Abstract
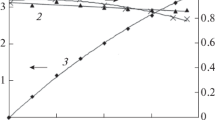
By substituting the ferrous to ferric oxidation for anodic oxygen evolution in an electrowinning cell, it is possible to reduce the cell voltage by about 1 V. However, it is then necessary to reduce the ferric back to ferrous and, depending on the circumstances, acid needs to be cogenerated. Various possible reductants are discussed, and experiments are described on the use of lignite and other carbonaceous materials to reduce the ferric ion. It was found that lignite was able to reduce the ferric ion,in situ in the electrowinning cell, but that the rate of reduction was compatible only with a maximum current density of about 40 Am-2. The efficiency was increased by periodically interrupting the current flow.
Similar content being viewed by others
References
W. Siemens: U.S. Patent Nos. 415, 576, 1889.
G. D. Van Arsdale:Trans. AIME, 1925, paper no. 1502-D, p. 17.
B.K. Loveday, R. A. Lynn, and J. P. Martin: South Africa NIM Report No. 1693, 1975, pp. 1-14.
K.K. Mishra and W. C. Cooper: inAnodes for Electrowinning, by D.J. Robinson and S.E. James, TMS-AIME, 1984, pp. 13-36.
D.J. Robinson:J. Met., 1984, vol. 36(1), pp. 43–47.
A. V. Cooke, J. P. Chilton, and D. J. Fray: inExtraction Metallurgy ’81, Proceedings of the IMM Conference in Extractive Metallurgy, London, Sept. 1981, pp. 430-41.
A. V. Cooke, J. P. Chilton, and D.J. Fray: inEnergy Reduction Techniques in Metal Electrochemical Processes, R. G. Bautista and R.J. Weseley, eds., TMS, Warrendale, PA, 1985, pp. 111–42.
C. H. Pitt and M. E. Wadsworth: Final Report to DOE Division of Industrial Energy Conservation, No. EM-78-S-07-1743, 1980.
Reference Electrodes, Theory and Practice, D. J.G. Ives and G. J. Janz, eds., Academic Press, New York, NY, 1961.
J. O’M. Bockris and A. K. N. Reddy:Modern Electrochem., Plenum Press, New York, NY, 1970, vols. 1 and 2.
P.M. Dhooge and S-M. Park:J. Electrochem. Soc., 1983, vol. 130, pp. 1539–42.
R. W. Coughlin and M. Farooque:Nature, 1979, vol. 279, pp. 301–03.
P.M. Dhooge and S-M. Park:J. Electrochem. Soc., 1983, vol. 130, pp. 1029–36.
G. Okada, V. Guruswamy, and J.O’M. Bockris:J. Electrochem. Soc., 1981, vol. 128, no. 10, pp. 2097–2102.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Cooke, A.V., Chilton, J.P. & Fray, D.J. An investigation of carbonaceous materials reducing ferric ions in aqueous solution. Metall Trans B 19, 709–717 (1988). https://doi.org/10.1007/BF02650190
Received:
Issue Date:
DOI: https://doi.org/10.1007/BF02650190