Summary
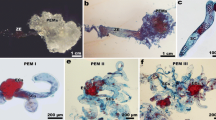
An anatomical study was carried out during the sequences of events which lead to the differentiation of secondary embryos ofCamellia reticulata cv ‘Mouchang’. Secondary embryogenesis can be induced by culturing somatic embryos on a modified Murashige and Skoog medium supplemented with 0.5 mg·liter−1 6-benzylaminopurine and 0.1 mg·liter−1 indole-3-butyric acid. After about 12 days of culture, globular-shaped secondary embryos became apparent, and by 18 to 20 days of culture cotyledonary stages were formed. Embryos developed mainly on the hypocotyl of primary embryos without an intermediate callus. Histologic monitoring revealed that secondary embryos apparently had a multicellular origin from embryogenic areas originating in both epidermal and subepidermal layers of the hypocotyl region. This morphogenetic competence is related to the presence, at the time of culture, of relatively undifferentiated cells in superfical layers of the primary embryo hypocotyl. Microcomputer image analysis was applied for quantifying cytological events associated with somatic embryogenesis. This method showed an increasing gradient in the nucleus-to-cell area ratio from differentiated cells passing through preembryogenic cells to embryogenic cells. The formation of embryogenic areas was preceded by accumulation of starch in the surrounding cortical cells. The cells underlying globular secondary embryos still contained abundant starch, but it declined as the secondary embryos developed.
Similar content being viewed by others
References
Ammirato, P. V. Recent progress in somatic embryogenesis. Newsletter IAPTC 57:2–16; 1989.
El Maâtaoui, M.; Espagnac, H.; Michaux-Ferrière, N. Histology of callogenesis and somatic embryogenesis induced in stem fragments of Cork Oak (Quercus suber) cultured in vitro. Ann. Bot. 66:183–190; 1990.
Féraud-Keller, C.; El Maâtaoui, M.; Gouin, O., et al. Embryogenése somatique chez trois espèces de chênes méditerranéens. Ann. Sci. For. 46(suppl):130s-132s; 1989.
Flinn, B. S.; Webb, D. T.; Newcomb, W. Morphometric analysis of reserve substances and ultrastructural changes during caulogenic determination and loss of competence of Eastern White pine (Pinus strobus) cotyledons in vitro. Can. J. Bot. 66:183–190; 1989.
Haccius, B. Question of unicellular origin of non-zygotic embryos in callus cultures. Phytomorphology 28:74–81; 1978.
Hartmann, H. T.; Kester, D. E.; Davies, F. T., Jr. Plant propagation: principles and practices, 5th ed. Englewood Cliffs, NJ: Prentice-Hall International Inc.; 1990:208–218.
Jensen, W. A. Botanical histochemistry. San Francisco, CA: W. H. Freeman; 1962:55–99.
Lu, C.; Vasil, I. K. Histology of somatic embryogenesis inPanicum maximum (Guinea grass). Am. J. Bot. 72:1989–1913; 1985.
Mangat, B. S.; Pelekis, M. K.; Cassells, A. C. Changes in the starch content during organogenesis in in vitro culturedBegonia rex stem explants. Physiol. Plant 79:267–274; 1990.
Margara, J. Bases de la multiplication végétative. Les méristèmes et l’organogenèse. Paris: Institut National de la Recherche Agronomique; 1982:25–29.
Mauseth, J. D. Plant anatomy. Menlo Park, CA: The Benjamin-Cummings Publ. Company; 1988:79–107.
Mazia, D.; Brewer, P. A.; Alfert, M. The cytochemical staining and measurement of protein with mercuric bromophenol blue. Biol. Bull. 104:57–67; 1953.
McClelland, M. T.; Smith, M. A. L. Vessel type, clousre, and explant orientation influence in vitro performance of five woody species. HortScience 25(7):797–800; 1990.
Merkle, S. A.; Wiecko, A. T. Regeneration ofRobinia pseudoacacia via somatic embryogenesis. Can. J. For. Res. 19:285–288; 1989.
Michaux-Ferrière, N.; Carron, M. Histology of early somatic embryogenesis inHevea brasiliensis: the importance of the timing of subculturing. Plant Cell Tissue Organ Cult. 19:243–256; 1989.
Michaux-Ferrière, N.; Bieysse, D.; Alvard, D., et al. Etude histologique de l’embryogenése somatique chez Coffeea arabica, induite par culture sur milieux uniques de fragments foliaires de gńotypes différents. Café Cacao Thé 33(4):207–217; 1989.
Muralidharan, E. M.; Gupta, P. K.; Mascarenhas, A. F. Plantlet production through high frequency somatic embryogenesis in long term cultures ofEucalyptus citriodora. Plant Cell Rep. 8:41–43; 1989.
Murashige, T.; Skoog, F. A revised medium for rapid growth and bioassays with tobacco tissue cultures. Physiol. Plant. 15:473–497; 1962.
Patel, K. R.; Thorpe, T. A. Histochemical examination of shoot initiation in cultured cotyledon explants of radiata pine. Bot. Gaz. 145(3):312–322; 1984.
Plata, E.; Viéitez, A. M. In vitro regeneration ofCamellia reticulata by somatic embryogenesis. J. Hortic. Sci. 65(6):707–714; 1990.
Profumo, P.; Gastaldo, P.; Dameri, R. M., et al. Histological study of calli and embryoids from leaf explants ofAesculus hisppocastanum L. J. Plant. Physiol. 126:97–103; 1986.
Quinn, J.; Simon, J. E.; Janick, J. Histology of zygotic and somatic embryogenesis in borage. J. Am. Soc. Hortic. Sci. 114(3):516–520; 1989.
Raj Bhansali, R. Somatic embryogenesis and regeneration of plantlets in pomegranate. Ann. Bot. 66:249–253; 1990.
Raj Bhansali, R.; Driver, J. A.; Durzan, D. J. Rapid multiplication of adventitious somatic embryos in peach and nectarine by secondary embryogenesis. Plant Cell Rep. 9:280–284; 1990.
Redenbaugh, K.; Slade, D.; Viss, P., et al. Encapsulation of somatic embryos in synthetic seed coats. HortScience 22(5):803–809; 1987.
Schwendiman, J.; Pannetier, C.; Michaux-Ferrière, N. Histology of somatic embryogenesis from leaf explants of the oil palmElaeis guineensis. Ann. Bot. 62:43–52; 1988.
Sharma, K. K.; Bhojwani, S. S. Histological aspects of in vitro root and shoot differentiation from cotyledon explants ofBrassica juncea (L.) Czern. Plant Sci. 69:207–214; 1990.
Sharp, W. R.; Sondhal, M. R.; Caldas, R. S., et al. The physiology of in vitro asexual embryogenesis. Hortic. Rev. 2:268–310; 1980.
Smith, M. A. L.; Spomer, L. A.; Meyer, M. J., et al. Non-invasive evaluation of growth during plant micropropagation. Plant Cell Tissue Organ Cult. 19:91–102; 1989.
Stamp, J. A. Somatic embryogenesis in cassava: the anatomy and morphology of the regeneration process. Ann. Bot. 59:451–459; 1987.
Stamp, J. A.; Henshaw, G. G. Secondary somatic embryogenesis and plant regeneration in cassava (Mannihot esculenta Grantz). Plant Cell Tissue Organ Cult. 10:227–233; 1987.
Thorpe, T. A. Organogenesis in vitro: structural, physiological and biochemical aspects, In: Vasil, I. K., ed. Perspectives in plant cell and tissure culture, Int. Rev. Cytol., Suppl. 11A. New York: Academic Press; 1980:71–111.
Thorpe, T. A. In vitro somatic embryogenesis. ISI atlas of science: Animal and Plant Science. 1:81–88; 1988.
Thorpe, T. A.; Murashige, T. Some histochemical changes underlying shoot initiation in tobacco callus cultures. Can. J. Bot. 48:277–285; 1970.
Tulecke, W.; McGranahan, G. Somatic embryogenesis and plant regeneration from cotyledons of walnut,Juglans regia L. Plant Sci. 40:57–63; 1985.
Viéitez, A. M.; Ballester, A.; García, M. T., et al. Starch depletion and anatomical changes during the rooting ofCastanea sativa Mill. cuttings. Sci. Hortic. 13:261–266; 1980.
Wann, S. R. Somatic embryogenesis in woody species. Hortic. Rev. 10:153–181; 1988.
Williams, E. G.; Maheswaran, G. Somatic embryogenesis: factors influencing coordinated behaviour of cells as an embryogenic group. Ann. Bot. 57:443–462; 1986.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Plata, E., Ballester, A. & Vieitez, A.M. An anatomical study of secondary embryogenesis inCamellia reticulata . In Vitro Cell Dev Biol - Plant 27, 183–189 (1991). https://doi.org/10.1007/BF02632214
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF02632214