Abstract
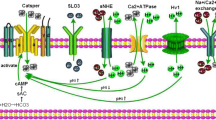
The effect of calcium channel blocker, verapamil (0.5–50 μM), has been studied in vitro in relation to certain spermatozoal functions in human ejaculates. Disruptive changes in the head and tail region of the spermatozoa, separation of heads from tails and coiling of the tail were observed. Motility was considerably reduced, while the pattern of motility also changed from rapid, linear progression to slow or sluggish linear or non-linear movement and finally to non-progressive motility, or even immotility. Verapamil significantly inhibited the influx of extracellular Ca2+. The study of kinetic effects further revealed a reduction in the maximum uptake velocity, but no change in the apparent substrate affinity constant. A highly significant decrease in Ca2+-dependent ATPase activity was also noted. In order to see whether this drug had any cytotoxic effect, presumably through lipid peroxides, thiobarbituric acid-reactive substances were measured. Verapamil produced an increase in the formation and release of malonyldialdehyde. The level of membrane cholesterol and phospholipid in the spermatozoa was also lowered considerably. The potential of such a calcium channel blocking agent in the designing of a male contraceptive programme is discussed.
Similar content being viewed by others
References
Alverez JG, Storey BT (1984) Assessment of cell damage caused by spontaneous lipid peroxidation in rabbit spermatozoa. Biol Reprod 30:323–331
Ashraf M, Peterson RN, Russell LD (1982) Activity and location of cation-dependent ATPase on the plasma membrane of boar spermatozoa. Biochem Biophys Res Commun 107:1273–1278
Ashraf M, Peterson RN, Russell LD (1984) Characterization of (Ca2++Mg2+) adenosine triphosphatase activity and calcium transport in boar sperm plasma membrane vesicles and their relation to phosphorylation of plasma membrane proteins. Biol Reprod 31:1061–1071
Babcock DF, Pfeiffer DR (1987) Independent elevation of cytosolic Ca2+ and pH of mammalian sperm by voltage-dependent and pH-dependent mechanisms. J Biol Chem 262: 15041–15047
Baccetti B (1975) Scanning electron microscopy of spermatozoa. In: Hayat MA (ed) Principle and techniques of electron microscopy, vol 3. Von Nostrand, New York
Barros C, Bedford JM, Franklin LE, Austin CR (1967) Membrane vesiculation as a feature of the mammalian acrosome reaction. J Cell Biol 34:C1-C5
Bartlett GR (1959) Phosphorus assay in the column chromatography. J Biol Chem 234:466–468
Bloj B, Zilversmit DB (1982) Heterogeneity of rabbit intestine brush border plasma membrane cholesterol. J Biol Chem 257:7608–7614
Breitbart H, Lardy HR (1987) Effect of verapamil and sulfhydryl reagents on calcium transport in bovine spermatozoa. Biol Reprod 36:658–663
Breitbart H, Stern B, Rubinstein S (1983) Calcium transport and Ca2+-ATPase activity in ram spermatozoa plasma membrane vesicles. Biochim Biophys Acta 728:349–355
Coronel CE, Lardy HA (1987) Characterization of Ca2+-uptake by guinea pig epididymal spermatozoa. Biol Reprod 37:1097–1101
Fiske CH, Subbarow Y (1925) The colorimetric determination of phosphorus. J Biol Chem 66:375–400
Folch J, Lees M, Sloane-Stanley GH (1957) A simple method for the isolation and purification of total lipids from nervous tissues. J Biol Chem 226:497–509
Fraser LR (1982) Ca2+ is required for mouse sperm capacitation and fertilization in vitro. J Androl 3:412–419
Fraser LR (1984) Mechanisms controlling mammalian fertilization. Oxford Rev Reprod Biol 6:174–225
Fraser LR, McIntyre K (1989) Calcium channel antagonists modulate the acrosome reaction but not capacitation in mouse spermatozoa. J Reprod Fertil 86:223–233
Girotti AW, Thomas JP (1984) Damaging effect of oxygen radicals on resealed erythrocyte ghosts. J Biol Chem 259:1744–1748
Girotti AW, Thomas JP, Jordon JE (1985) Lipid peroxidation in erythrocyte ghosts: sensitization of the membranes towards ascorbate and superoxide induce peroxidation and lysis. Biophys J 236:238–240
Green DPL (1978) Induction of the acrosome reaction in guinea pig sperm by divalent metal cation ionophore A23187. J Cell Sci 32:137–151
Guraya SS (1987) Biology of spermatogenesis and spermatozoa in mammals. Springer, Berlin Heidelberg New York, pp 284–285
Hafez ESE (1976) Human semen and fertility regulation in men. Mosby, New York, p 65
Hyne RV, Higginson RE, Kohlman D, Lopata A (1984) Sodium requirement for capacitation and membrane fusion during the guinea-pig sperm acrosome reaction. J Reprod Fertil 70:83–94
Jones R, Mann T (1977) Toxicity of exogenous fatty acid peroxides towards spermatozoa. J Reprod Fertil 50:255–259
Jones R, Mann T (1977) Damage to spermatozoa by peroxidation of endogenous phospholipids. J Reprod Fertil 50:261–270
Kanwar U, Batla A, Sanyal S, Minocha R, Majumdar S, Ranga A (1989) Gossypol inhibition of Ca2+-uptake and Ca2+-ATPase in human ejaculated spermatozoal plasma membrane vesicles. Contraception 39:431–445
Kazazoglou T, Schackmann RW, Fosset M, Shapiro BM (1985) Calcium channel antagonists inhibit the acrosome reaction and bind to plasma membranes of sea urchin sperm. Proc Natl Acad Sci USA 82:1462–1464
Lees M, Paxman S (1972) Modification of the Lowry procedure for the analysis of prote-olipid protein. Anal Biochem 47:184–192
Lehrminier M, Alvarado F (1981) Virtual elimination of the intestinal sugar transport kinetics by use of the tissue accumulation method at appropriate shaking rates. Pflugers Arch 389:155–158
Mann T, Mann CL (1981) In: Male reproductive function and semen, Springer, Berlin Heidelberg New York, pp 195–268
Marinetti GV (1982) Chromatographic separation, identification and analysis of phospholipids. J Lipid Res 3:1–5
Meizel S (1984) The importance of hydrolytic enzymes to an exocytotic event, the mammalian sperm acrosome reaction. Biol Rev 59:125–157
Renton KW (1985) Inhibition of hepatic microsomal drug metabolism by the calcium channel blockers diltiazem and verapamil. Toxicology 34:2549–2553
Roldan ERS, Fleming AD (1989) Is a Ca2+-ATPase involved in Ca2+ regulation during capacitation and the acrosome reaction of guinea pig spermatozoa. J Reprod Fertil 85:297–308
Rufo GA, Schoff PK, Lardy HA (1984) Regulation of calcium content in bovine spermatozoa. J Biol Chem 259:2547–2552
Schackmann RW, Eddy EM, Shapiro BM (1978) The acrosome reaction ofStrongylocentrotus purpuratus sperm. Ion requirements and movements. Dev Biol 65:483–495
Seth SD, Maulik SK, Gupta YK (1987) Calcium and calcium channel blockers: basic and clinical considerations. J Exp Biol 25:729–741
Singh JP, Babcock DF, Lardy HA (1978) Increased calcium-ion influx is a component of capacitation of spermatozoa. Biochem J 172:549–556
Singh JP, Babcock DF, Lardy HA (1980) Induction of accelerated acrosome reaction in guinea pig sperm. Biol Reprod 22:566–570
Talbot P, Summers RG, Hylander BL, Keough EM, Franklin LE (1976) The role of calcium in the acrosome reaction: an analysis using the ionophore A23187. J Exp Zool 198:383–392
Triggle DJ, Janis RA (1987) Calcium channel ligands. Annu Rev Pharmacol Toxicol 27: 347–369
WHO laboratory manual for the examination of human semen and semen-cervical mucus interaction (1992). Cambridge University Press, Cambridge, pp 3–18
Yanagimachi R (1981) Mechanisms of fertilization in mammals. In: Mastroianni L Jr, Biggerss JD (eds) Fertilization and embryonic development in vitro. Plenum Press, New York, pp 81–182
Yanagimachi R, Usui N (1974) Calcium dependence of the acrosome reaction and activation of guinea pig spermatozoa. Exp Cell Res 89:161–174
Yuli I, Wilbrandt W, Shinitsky M (1981) Glucose transport through cell membrane of modified lipid fluidity. Biochemistry 20:4250–4256
Zlatkis A, Zak B, Boyle AJ (1953) A new method for the direct determination for serum cholesterol. J Lab Clin Med 41:486–492
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Anand, R.J.K., Kanwar, U. & Sanyal, S.N. Calcium channel antagonist verapamil modulates human spermatozoal functions. Res. Exp. Med. 194, 165–178 (1994). https://doi.org/10.1007/BF02576377
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF02576377