Abstract
Background
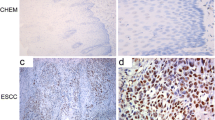
It would be of considerable benefit to patients with esophageal cancer to be able to predict the effect of CRT before therapy, because critical side effects could be avoided and the therapeutic cost of CRT-resistant cases could be reduced. One of the biological parameters with the potential to indicate radioresponse is the DNA double-strand break repair enzyme DNA-PKcs. This study aims to clarify the correlation between DNA-PKcs expression and CRT effect.
Methods
Sixty-seven patients with progressive esophageal cancer treated with CRT were included in this study. The relationship between the expression of DNA-PKcs and the effect of CRT was examined by using immunohistochemistry. The relationships between DNA-PKcs expression, clinicopathologic parameters, and CRT effect were investigated statistically.
Results
A significant correlation was found between the expression of DNA-PKcs and the effect of CRT (P=.0149). The high-DNA-PKcs expression group showed greater therapeutic sensitivity than the low-expression croup. Clinicopathologic factors had no relationship with DNA-PKcs expression or CRT effect.
Conclusions
This study suggests that high expression of DNA-PKcs correlates with CRT effect. DNA-PKcs expression could, therefore, be useful for predicting the effect of CRT. In addition, these results may make it possible to plan therapy taking patients’ quality of life into consideration.
Similar content being viewed by others
References
Parker SL, Tong T, Bolden S, Wingo PA. Cancer statistics, 1993.CA Cancer J Clin 1996;46:5–27.
Bilingsley KG, Maynard C, Schwartz DL, Dominitz JA. The use of trimodality therapy for the treatment of operable esophageal carcinoma in the veteran population. Cancer 2001;95:1272–80.
Arnott SJ, Duncan W, Kerr GR, et al. Low dose preoperative radiotherapy for carcinoma of the esophagus: results of a randomized clinical trial.Radiother Oncol 1992;24:108–13.
Gignoux M, Roussel A, Paillot B, et al. The value of preoperative radiotherapy in esophageal cancer: results of a study of the E.O.R.T.C..World J Surg 1987;11:426–32.
Launois B, Delarue D, Campion JP, Kerbaol M. Preoperative radiotherapy for carcinoma of the esophagus.Surg Gynecol Obstet 1981;153:690–2.
Teniere P, Hay JM, Fingerhut A, Fagniez PL. Postoperative radiation therapy does not increase survival after curative resection for squamous cell carcinoma of the middle and lower esophagus as shown by a multicenter controlled trial. French University Association for Surgical Research.Surg Gynecol Obstet 1991;173:123–30.
Matsuyama A, Inoue H, Shibuta K, et al. Hepatoma-derived growth factor is associated with reduced sensitivity to irradiation in esophageal cancer.Cancer Res 2001;61:5714–7.
FitzGerald TJ, Henault S, Sakakeeny M, et al. Expression of transfected recombinant oncogenes increases radiation resistance of clonal hematopoietic and fibroblast cell lines selectively at clinical low dose rate.Radiat Res 1990;122:44–52.
Hallahan DE, Dunphy E, Virudachalam S, Sukhatme VP, Kufe DW, Weichselbaum RR. C-jun and Egr-1 participate in DNA synthesis and cell survival in response to ionizing radiation exposure.J Biol Chem 1995;270:30303–9.
Sklar MD. The ras oncogenes increase the intrinsic resistance of NIH 3T3 cells to ionizing radiation.Science 1988;239: 645–7.
Bernhard EJ, Kao G, Cox AD, et al. The farnesyltransferase inhibitor FTI-277 radiosensitizes H-ras-transformed rat embryo fibroblasts.Cancer Res 1996;56:1727–30.
Kasid U, Pfeifer A, Weichselbaum RR, Dritschilo A, Mark GE. The rat oncogene is associated with a radiation-resistant human laryngeal cancer.Science 1987;237:1039–41.
Santucci MA, Anklesaria P, Anderson SM, et al. The v-src oncogene may not be responsible for the increased radioresistance of hematopoietic progenitor cells expressing v-src.Radiat Res 1992; 129:297–303.
Kitada S, Krajewski S, Miyashita T, Krajewska M, Reed JC. Gamma-radiation induces upregulation of Bax protein and apoptosis in radiosensitive cells in vivo.Oncogene 1996;12:187–92.
Cohen Jonathan E, Toulas C, Monteil S, et al. Radioresistance induced by the high molecular forms of the basic fibroblast growth factor is associated with an increased G2 delay and a hyperphosphorylation of p34CDC2 in HeLa cells.Cancer Res 1997;57:1364–70.
Ding I, Huang K, Snyder ML, et al. Tumor growth and tumor radiosensitivity in mice given myeloprotective doses of fibroblast growth factors.J Natl Cancer Inst 1996;88:1339–404.
Biard DS, Martin M, Rhun YL, et al. Concomitant p53 gene mutation and increased radiosensitivity in rat lung embryo epithelial cells during neoplastic development.Cancer Res 1994;54:3361–64.
Tsang NM, Nagasawa H, Li C, Little JB. Abrogation of p53 function by transfection of HPV16 E6 gene enhances the resistance of human diploid fibroblasts to ionizing radiation.Oncogene 1995;10:2403–8.
Brachman DG, Beckett M, Graves D, Haraf D, Vokes E, Weichselbaum RR. p53 mutation does not correlate with radiosensitivity in 24 head and neck cancer cell lines.Cancer Res 1993;53:3667–9.
Takeno S, Noguchi T, Takahashi Y, Kikuchi R, Uchida U. Yokoyama Y. Immunohistochemical and clinicopathologic analysis of response to neoadjuvant therapy for esophageal squamous cell carcinoma.Dis Esoph 2001;14:149–54.
Carter T, Vancurova I, Sun I, Lou W, Deleon S. A DNA-activated protein kinase from HeLa cell nuclei.Mol Cell Biol 1990;10:6460–71.
Smith GC, Jackson SP. The DNA-dependent protein kinase.Genes Dev 1999;13:916–34.
Sakata K, Matsumoto Y, Satoh M, et al. Clinical studies of immunohistochemical staining of DNA-dependent protein kinase in oropharyngeal and hypopharyngeal carcinomas.Radiat Med 2001;19:93–2.
Japanese Society for Esophageal Disease.Guidelines for Clinical and Pathologic Studies on Carcinoma of the Esophagus. 9th ed. Tokyo: Kanehara & Co Ltd, 2001:68–72.
Forastiere AA, Orringer MB, Petez-Tamayo C, Urba SG, Zahurak M. Preoperative chemoradiation followed by transhiatal esophagectomy for carcinoma of the esophagus: final report.J Clin Oncol 1993;11:1118–23.
Coia LR. Chemoradiation as primary management of esophageal cancer.Semin Oncol 1994;21:483–92.
Daly JM, Karnell LH, Menck HR. National cancer data base report on esophageal carcinoma.Cancer 1996;78:1820–8.
Meneu-Diaz JC, Blazquez LA, Vincent E, et al. The role of multimodality therapy for resectable esophageal cancer.Am J Surg 2000;89:946–54.
Adham M, Baulieux J, de la Roche de Bransat E, et al. Combined chemotherapy and radiotherapy followed by surgery in the treatment of patients with squamous cell carcinoma of the esophagus.Cancer 2000;89:946–54.
Bjork-Eriksson T, West C, Nilsson A, et al. The immunohistochemical expression of DNA-PKcs and Ku (p70/p80) in head and neck cancers: relationships with radiosensitivity.Int J Radiat Oncol Biol Phys 1999;45:1005–10.
Jeggo PA. Identification of genes involved in repair of DNA double-strand breaks in mammalian cells.Radiat Res 1998;150(5 Suppl):S80–91.
Featherstone C, Jackson SP. DNA repair: the Nijmegen breakage syndrome protein.Curr Biol 1998;8:R622–5.
Less-Miller SP, Chen YR, Anderson CW. Human cells contain a DNA activated protein kinase that phosphorylates simian virus 40 T antigen, mouse p53 and the human Ku autoantigen.Mol Cell Biol 1990;10:6472–81.
Gottlieb TM, Jackson SP. The DNA-dependent protein kinase. Requirement for DNA ends and association with Ku antigen.Cell 1993;72:131–42.
Wang S, Guo M, Ouyang H, et al. The catalytic subunit of DNA-dependent protein kinase selectively regulates p53-dependent apoptosis but not cell-cycle arrest.Proc Natl Acad Sci U S A 2000;97:1584–8.
Woo RA, McLure KG, Lees-Miller SP, Rancourt DE, Lee PWK. DNA-dependent protein kinase acts upstream of p53 in response to DNA damage.Nature 1998;394:700–4.
Siliciano JD, Canman CE, Taya Y, Sakaguchi K, Apella E, Kastan MB. DNA damage induces phosphorylation of the amino terminus of p53.Genes Dev 1997;11:3471–81.
Schmitt CA, Lowe SW. Apoptosis and therapy.J Pathol 1999;187:127–37.
Ribeito U, Finkelstein SD, Safatle-Riberio AV, Landreneu RJ, Clarke MR, Bakker A. p53 sequence analysis predicts treatment response and outcome of patients with esophageal carcinoma.Cancer 1998;83:7–18.
Nemoto K, Ariga H, Kakuto Y, et al. Radiation therapy for loco-regionally recurrent esophageal cancer after surgery.Radiother Oncol 2001;61:165–8.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Noguchi, T., Shibata, T., Fumoto, S. et al. DNA-PKcs expression in esophageal cancer as a predictor for chemoradiation therapeutic sensitivity. Annals of Surgical Oncology 9, 1017–1022 (2002). https://doi.org/10.1007/BF02574522
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF02574522