Summary
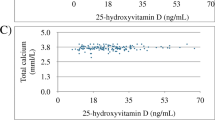
The common marmoset, a New World monkey, requires a large amount of vitamin D3 to maintain its normal growth. This monkey is reported to have an end-organ resistance to 1α,25-dihydroxyvitamin D3 (1α, 25(OH)2D3). In this study, the bone morphology of marmosets fed a high vitamin D3 diet (intake of vitamin D3, 110 IU/day/100 g of body weight) was compared by X-ray and histological examinations with that of rhesus monkeys (Old World monkey) fed a normal diet (intake of vitamin D3, 5 IU/day/100 g of body weight). Three of 20 marmosets were found by X-ray examination to have osteomalacic changes in their bones despite the high daily intake of vitamin D3, whereas none of the 5 rhesus monkeys showed any signs of osteomalacica. Osteomalacic marmosets had distinct incrases in osteoid surface, relative osteoid volume, and active osteoclastic bone resorption, whereas nonosteomalacic marmosets had no increase in osteoid tissues in their bones. None of the marmosets, either osteomalacic or nonosteomalacic, was hypercalcemic despite the extremely high circulating levels of 1α,25(OH)2D3. However, the serum 25-hydroxy vitamin D3 (25OHD3) and 24R,25-dihydroxyvitamin D3 (24R,25(OH)2D3) levels were significantly lower in the osteomalacic than in the nonosteomalacic marmosets. These results suggest that the marmoset is likely to exhibit osteomalacic bone changes despite the high daily intake of vitamin D3. These changes resemble those in vitamin D-dependent rickets, type II.
Similar content being viewed by others
References
Shinki T, Shiina Y, Takahashi N, Tanioka Y, Koizumi H, Suda T (1983) Extremely high circulating levels of 1α,25-dihydroxyvitamin D3 in the marmoset, a New World monkey. Biochem Biophys Res Commun 114:452–457
Suda T, Takahashi N, Shinki T, Yamaguchi A, Tanioka Y (in press) The common marmoset as an animal model for vitamin D-dependent rickets, type II. Adv Exp Med Biol
Takahashi N, Suda S, Shinki T, Horiuchi N, Shiina Y, Tanioka Y, Koizumi H, Suda T (1985) The mechanism of endorgan resistance to 1α,25-dihydroxycholecalciferol in the common marmoset. Biochem J 227:555–563
Liberman UA, Grange D, Marx SJ (1985) Low affinity of the receptor for 1α,25-dihydroxyvitamin D3 in the marmoset, a New World monkey. FEBS Letter 182:385–388
Adams JS, Gacad MA, Baker AJ, Kheun G (1985) Diminished internalization and action of 1,25-dihydroxyvitamin D3 in dermal fibroblasts cultured from New World primates. Endocrinol 116:2523–2527
Brooks MH, Bell NH, Love L, Stern PH, Orfei E, Queener SF, Hamstra AJ, DeLuca HF (1978) Vitamin D-dependent rickets, type II. Resistance of target organs to 1α,25-dihydroxyvitamin D. N Engl J Med 298:996–999
Zerwekk JE, Glass K, Jowsey J, Charles YC (1979) An unique form of osteomalacia associated with end-organ refractoriness to 1α,25-dihydroxyvitamin D and apparent defective synthesis of 25-hydroxyvitamin D. J Clin Endocrinol Metab 49:171–175
Liberman UA, Samuel R, Halabe A, Kauli R, Edelstein S, Weisman Y, Papapoulos SE, Clemens TL, Fraher LJ. O'Riordan JLH (1980) End-organ resistance to 1α,25-dihydroxycholecalciferol. Lancet i:504–507
Balsan SM, Garabedian UA, Liberman UA, Eli C, Bourdeau A, Guillozo H, Grimberg R, LeDeunff MJ, Lieberherr M, Gumbaud P, Broyer M, Marx SJ (1983) Rickets and alopecia with resistance to 1,25-dihydroxyvitamin D: two different clinical courses with two different cellular defects. J Clin Endocrinol Metab 57:803–811
Marx SJ (1984) Resistance to vitamin D. In: L Kumar RJ (ed) Vitamin D: basic and clinical aspects. Martinus Nijhoff Publishing, Boston, Massachusetts.
Eil C, Liberman UA, Rosen JF, Marx SJ (1981) A cellular defect in hereditary vitamin D-dependent rickets, type II: defective nuclear uptake of 1,25-dihydroxyvitamin D in cultured skin fibroblasts. N Engl J Med 304:1588–1591
Liberman UA, Eil C, Marx J (1983) Resistance to 1,25-dihydroxyvitamin D. Association with heterogeneous defects in cultured skin fibroblasts. J Clin Invest 71:192–200
Pike JW, Dokoh S, Hussler MR, Liberman UA, Marx SJ, Eil C (1984) Vitamin D3-resistant fibroblasts have immunoassayable 1,25-dihydroxyvitamin D3 receptors. Science 224:879–881
Liberman UA, Eil C, Holst P, Rosen JF, Marx SJ (1983) Hereditary resistance to 1,25-dihydroxyvitamin D: defective function of receptors for 1,25-dihydroxyvitamin D in cells cultured from bone. J Clin Endocrinol Metab 57:958–962
Yoshiki S, Ueno T, Akita T, Yamanouchi M (1983) Improved procedure for histological identification of osteoid matrix in decalcified bone. Stain Technol 58:85–89
Fiske CH, Subbarow Y (1925) The colorimetric determination of phosphorus. J Biol Chem 66:357–400
Kano K, Yoshida H, Yata J, Abe E, Tanabe R, Suda T (1979) An assay method for separately measuring metabolites of vitamin D3 and those presumed to be derived from vitamin D2. J Nutr Sci Vitaminol 25:351–360
Jowsey J (1974) Bone histology and hyperparaphyroidism. Clin Endocrinol Metab 3:267–284
Ornoy A, Goodwin D, Noff D, Edelstein S (1978) 24,25-dihydroxyvitamin D is a metabolite of vitamin D essential for bone formation. Nature 276:517–519
Endo H, Kiyoki M, Kawashima K, Naruchi T, Hashimoto Y (1980) Vitamin D3 metabolites and PTH synergistically stimulate bone formation of chick embryonic femur in vitro. Nature 286:262–264
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Yamaguchi, A., Kohno, Y., Yamazaki, T. et al. Bone in the marmoset: A resemblance to vitamin D-dependent rickets, type II. Calcif Tissue Int 39, 22–27 (1986). https://doi.org/10.1007/BF02555736
Received:
Revised:
Issue Date:
DOI: https://doi.org/10.1007/BF02555736