Summary
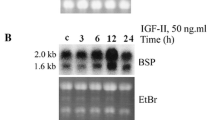
The synthesis of matrix Gla protein (MGP) and bone Gla protein (BGP) have been shown to be mutually exclusive in all osteosarcoma cell lines investigated. In the cell lines that produce the respective proteins, synthesis is stimulated by 1,25-dihydroxyvitamin D3(1,25(OH)2D3) within the first several hours of hormone treatment. In the present studies we have investigated the effects of longer-term treatment with 1,25(OH)2D3 in the ROS 17/2 cell line, a cell line that synthesizes BGP constitutively but does not synthesize MGP. In agreement with earlier studies, the rate of BGP synthesis increases within 8 hours of hormone treatment, is maximal by 24 hours, and remains at the maximal rate through 48 hours of 1,25(OH)2D3 treatment. The present study is the first to report that the rate of BGP secretion at times beyond 48 hours declines to that of control cultures despite the continued administration of 1,25(OH)2D3, and that MGP synthesis is induced in ROS 17/2 cells by 48 hours of 1,25(OH)2D3 treatment. At this time, MGP mRNA could be detected by northern blot analysis and MGP secretion could be demonstrated by radioimmunoassay of culture medium. Both the level of MGP message per unit total RNA and the rate of MGP secretion into culture medium increased steadily between 2 and 6 days of 1,25(OH)2D3 treatment. The MGP synthesized by the 1,25(OH)2D3-treated ROS 17/2 cells was identical to that found in bone by northern blot analysis of message and by western blot analysis of the media antigen. Halfmaximal induction of MGP synthesis was obtained with 0.3 nM 1,25(OH)2D3, a 60-fold higher dosage than was required for the half maximal stimulation of BGP synthesis in these cells. Treatment of ROS 17/2 cells with 24,24-F21,25(OH)2D3 suggests that the observed difference in dose dependence is not due to an increased rate of hormone catabolism.
Similar content being viewed by others
References
Hauschka PV, Lian JB, Gallop PM (1975) Direct identification of the calcium-binding amino acid, γ-carboxyglutamate, in mineralized tissue. Proc Natl Acad Sci USA 72:3925–3929
Price PA, Otsuka AS, Poser JW, Kristaponis J, Raman N (1976) Characterization of a γ-carboxyglutamic acidcontaining protein from bone. Proc Natl Acad Sci USA 73:1447–1451
Price PA, Urist MR, Otawara Y (1983) Matrix gla protein, a new γ-carboxyglutamic acid-containing protein which is associated with the organic matrix of bone. Biochem Biophys Res Comm 117:765–771
Price PA, Williamson MK (1985) Primary structure of bovine matrix gla protein, a new vitamin K-dependent bone protein. J Biol Chem 260:14971–14975
Poser JW, Price PA (1979) A method for decarboxylation of γ-carboxyglutamic acid in proteins. J Biol Chem 254:431–436
Otwara Y, Price PA (1986) Developmental apperance of matrix gla protein during calcification in the rat. J Biol Chem 261:10828–10832
Price PA, Williamson MK, Haba T, Dell RB, Jee WSS (1982) Excessive mineralization with growth plate closure in rats on chronic warfarin treatment. Proc Natl Acad Sci USA 79:7734–7738
Nishimoto SK, Price PA (1980) Secretion of the vitamin K-dependent protein of bone by rat osteosarcoma cells. Evidence from an intracellular precursor. J Biol Chem 255:6579–6583
Fraser JD, Otawara Y, Price PA (1988) 1,25-Dihydroxyvitamin D3 stimulates the synthesis of matrix gla protein by osteosarcoma cells. J Biol Chem 263:911–916
Abe E, Miyaura C, Sakagami H, Takeda M, Konno K, Yamazaki T, Yoshiki S, Suda T (1981) Differentiation of mouse myeloid leukemia cells induced by 1 alpha, 25-dihydroxyvitamin D3. Proc Natl Acad Sci USA 78:4990–4994
Tanaka H, Abe E, Miyaura C, Kuribayashi T, Konno K, Nishi Y, Suda T (1982) 1 alpha, 25-dihydroxycholecalciferol and a human myeloid leukemia cell line (HL-60). Biochem J 204:713–719
Bar-Shavit Z, Teitelbaum SL, Reitsma P, Hall A, Pegg LE, Trial J, Kahn AJ (1983) Induction of monocytic differentiation and bone resorption by 1,25-dihydroxyvitamin D3. Proc Natl Acad Sci USA 80:5907–5911
Dodd RC, Cohen MS, Newman SL, Gray TK (1983) Vitamin D metabolites change the phenotype of monoblastic U937 cells. Proc Natl Acad Sci USA 80:7538–7541
Mangelsdorf DJ, Koeffler HP, Donaldson CA, Pike WP, Haussler MR (1984) 1,25-Dihydroxyvitamin D3-induced differentiation in a human promyelocytic leukemia cell line (HL-60): receptor-mediated maturation to macrophage-like cells. J Cell Biol 98:391–398
Hosomi J, Hosoi J, Abe E, Suda T, Kuroki T (1983) Regulation of terminal differentiation of cultured mouse epidermal cells by 1 alpha, 25-dihydroxyvitamin D3. Endocrinology 113:1950–1957
MacLaughlin JA, Gange W, Taylor D, Smith E, Holick MF (1985) Cultured psoriatic fibroblasts from involved and uninvolved sites have a partial but not absolute resistance to the proliferation-inhibition activity of 1,25-dihydroxyvitamin D3. Proc Natl Acad Sci USA 82:5409–5412
Majeska RJ, Rodan GA (1982) The effect of 1,25(OH)2D3 on alkaline phosphatase in osteoblastic osteosarcoma cells. J Biol Chem 257:3362–3365
Dokoh S, Donaldson CA, Haussler MR (1984) Influence of 1,25-dihydroxyvitamin D3 on cultured osteogenic sarcoma cells: correlation with the 1,25-dihydroxyvitamin D3 receptor. Cancer Res 44:2103–2109
Majeska RJ, Rodan SB, Rodan GA (1980) Parathyroid hormone-responsive clonal cell lines from rat osteosarcoma. Endocrinology 107:1494–1503
Price PA, Nishimoto SK (1980) Radioimmunoassay for the vitamin K-dependent protein of bone and its discovery in plasma. Proc Natl Acad Sci USA 77:2234–2238
Laemmli UK (1970) Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 227:680–685
Towbin A, Staehelin T, Gordon J (1979) Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci USA 76:4350–4354
Chirgwin JM, Przbyla AE, MacDonald RJ, Rutter WJ (1979) Isolation of biologically active ribonucleic acid from sources enriched in ribonuclease. Biochemistry 18:5294–5299
Seed B (1982) Attachment of nucleic acids to nitrocellulose and diazonium-substituted supports. In: Setlow JK, Hollaender A (eds) Genetic engineering: Principles and methods, vol 4, Plenum Press, New York, p 91–102
Maniatis T, Fritsch EF, Sambrook J (1982) Molecular cloning: A laboratory manual. Cold Spring Harbor Laboratory, Cold Spring Harbor, New York, p 203–206
Pan LC, Price PA (1985) The propeptide of rat bone γ-carboxyglutamic acid protein shares homology with other vitamin K-dependent protein precursors. Proc Natl Acad Sci USA 82:6109–6113
Price PA, Fraser JD, Metz-Virca G (1987) Molecular cloning of matrix gla protein: implications for substrate recognition by the vitamin K-dependent γ-carboxylase. Proc Natl Acad Sci USA 84:8335–8339
Nudel U, Katcoff D, Zakut R, Shani M, Carmon Y, Finer M, Czosnek H, Ginsburg I, Yaffe D (1982) Isolation and characterization of rat skeletal muscle and cytoplasmic actin genes. Proc Natl Acad Sci USA 79:2763–2767
Rigby PW, Dieckman M, Rhodes C, Berg P (1977) Labeling deoxyribonucleic acid to high specific activity in vitro by nick translation with DNA polymerase I. J Mol Biol 113:237–251
Singh L, Jones KW (1984) The use of heparin as a simple cost-effective means of controlling background in nucleic acid hybridization procedures. Nucleic Acids Res 12:5627–5638
Price PA, Baukol SA (1980) 1,25-Dihydroxyvitamin D3 increases synthesis of the vitamin K-dependent bone protein by osteosarcoma cells. J Biol Chem 255:11660–11663
Lohnes D, Jones G (1987) Side chain metabolism of vitamin D3 in osteosarcoma cell line UMR-106. Characterization of products. J Biol Chem 262:14394–14401
Tanaka Y, DeLuca HF, Schnoes HK, Ikekawa N, Kobayashi Y (1980) 24,24-difluoro-1,25-dihydroxyvitamin D3: in vitro production, isolation and biological activity. Arch Biochem Biophys 199:473–478
Halloran BP, DeLuca HF, Barthell E, Yamada S, Ohmori M, Takayama H (1981) An examination of the importance of 24-hydroxylation to the function of vitamin D during early development. Endocrinology 108:2067–2071
Miller SC, Halloran BP, DeLuca HF, Yamada S, Takayama H, Jee WSS (1981) Studies on the role of 24-hydroxylation of vitamin D in the mineralization of cartilage and bone of vitamin D-deficient rats. Calcif Tissue Int 33:489–497
Eisman JA, DeLuca HF (1977) Intestinal 1,25-dihydroxy-vitamin D3 binding protein: specificity of binding. Steroids 30:245–257
Haussler MR (1986) Vitamin D receptors: nature and function. Ann Rev Nutr 6:527–562
Costa EM, Hirst MA, Feldman D (1985) Regulation of 1,25-dihydroxyvitamin D3 receptors by vitamin D analogs in cultured mammalian cells. Endocrinology 117:2203–2210
Hirst M, Feldman D (1983) Regulation of 1,25(OH)2 vitamin D3 receptor content in cultured LLC-PK1 kidney cells limits hormonal responsiveness. Biochem Biophys Res Comm 116:121–127
Sher E, Frampton RJ, Eisman JA (1985) Regulation of the 1,25-dihydroxyvitamin D3 receptor by 1,25-dihydroxyvitamin D3 in intact human cancer cells. Endocrinology 116:971–977
Pan LC, Price PA (1987) Ligand-dependent regulation of the 1,25-dihydroxyvitamin D3 receptor in rat osteosarcoma cells. J Biol Chem 262:4670–4675
Thompson CC, Weinberger C, Lebo R, Evans RM (1987) Indentification of a novel thyroid hormone receptor expressed in the mammalian central nervous system. Science 237:1610–1614
Pierce EA, Dame MC, DeLuca HF (1987) Size and charge of the functional 1,25-dihydroxyvitamin D receptor in procine intestine. J Biol Chem 262:17092–17099
Pike JW, Sleartor NM (1985) Hormone-dependent phosphorylation of the 1,25-dihydroxyvitamin D3 receptor in mouse fibroblasts. Biochem Biophys Res Commun 131:378–385
Housley PR, Pratt WB (1983) Direct demonstrations of glucocorticoid receptor phosphorylation by intact L-cells. J Biol Chem 258:4630–4635
Dougherty JJ, Puri RK, Toft DO (1982) Phosphorylation in vivo of chicken oviduct progesterone receptor. J Biol Chem 257:14226–14230
Auricchio F, Migliaccio A, Castoria G, Lastoria S, Rotondi A (1982) Evidence that in vivo estradiol receptor translocated into nuclei is dephosphorylated and released into cytoplasm. Biochem Biophys Res Commun 106:149–157
Smith RG, Clarke SG, Zalta E, Taylor RN (1979) Two estrogen receptors in reproductive tissue. J Steroid Biochem 10:31–35
McNaught RW, Smith RG (1986) Characterization of a second estrogen receptor species in chick oviduct. Biochemistry 25:2073–2081
McNaught RW, Raymoure WJ, Smith RG (1986) Receptor interconversion model of hormone action. I. ATP-mediated conversion of estrogen receptors from a high to lower affinity state and its relationship to antiestrogen action. J Biol Chem 261:17011–17017
Rodan GA, Rodan SB (1984) Expression of the osteoblastic phenotype. In: Peck WA (ed) Bone and mineral research annual 2. Elsevier, Amsterdam, p 244–285
Manolagas SC, Burton DW, Deftos LJ (1981) 1,25-dihydroxyvitamin D3 stimulates the alkaline phosphatase activity of osteoblast-like cells. J Biol Chem 256:7115–7117
Price PA, Sloper SA (1983) Concurrent warfarin treatment further reduces bone mineral levels in 1,25-dihydroxyvitamin D3-treated rats. J Biol Chem 258:6004–6007
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Fraser, J.D., Price, P.A. Induction of matrix gla protein synthesis during prolonged 1,25-Dihydroxyvitamin D3 treatment of osteosarcoma cells. Calcif Tissue Int 46, 270–279 (1990). https://doi.org/10.1007/BF02555007
Received:
Revised:
Issue Date:
DOI: https://doi.org/10.1007/BF02555007