Abstract
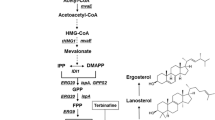
Squalene synthetase (EC 2.5.1.21) and squalene epoxidase (EC 1.14 99.7) activities have been measured in cell-free extracts of wild type yeast grown in aerobic and semianaerobic conditions as well as in sterol-auxotrophic mutant strains grown aerobically. The results show that both enzymes are induced resulting in an almost two- to five-fold increase in enzymatic activities in mutant strains containing limited sterol amounts and are repressed in the wild type strain cultured in anaerobiosis in excess of sterol. The results show also that squalene epoxidase is repressed by lanosterol, and that the mevalonic acid pool may regulate squalene synthetase levels.
The large change in the activities of the two enzymes, depending on the sterol needs of the cells, as well as their low specific activities in comparison with those of the enzymes involved in the early stages of sterol synthesis strongly suggests that squalene synthetase and squalene epoxidase are of importance in regulating the amount of sterol synthesized by yeast.
Similar content being viewed by others
Abbreviations
- DTT:
-
dithiothreitol
- erg:
-
mutant gene involved in ergosterol biosynthesis
- FAD:
-
flavin adenine dinucleotide
- HMG1:
-
HMG2, genes
- HMG-CoA:
-
hydroxy methyl glutaryl coenzyme A
- LDL:
-
low-density lipoprotein
- TLC:
-
thin-layer chromatography
- tRNA:
-
transfer ribonucleic acid
References
Brown, M.S., and Goldstein, J.L. (1980)J. Lipid Res. 21, 505–517.
Basson, M.E., Thorness, M., and Rine, J. (1986)Proc. Natl. Acad. Sci. USA 83, 5563–5567.
Servouse, M., and Karst, F. (1986)Biochem. J. 240, 541–547.
Karst, F., and Lacroute, F. (1977)Mol. Gen. Genet. 154, 259–277.
Servouse, M., Mons, N., Baillargeat, J.L., and Karst, F. (1984)Biochem. Biophys. Res. Commun. 123, 424–430.
Trocha, P.J., and Sprinson, D.B. (1976)Arch. Biochem. Biophys. 174, 45–51.
Warburg, G.F., Wakeel, M., and Wilton, D.C. (1982)Lipids 17, 230–234.
Klein, H.P. (1955)J. Bacteriol. 69, 620–627.
Boll, M., Löwel, M., Still, J., and Berndt, J. (1975)Eur. J. Biochem. 54, 435–444.
M'Baya, B., and Karst, F. (1987)Biochem. Biophys. Res. Commun. 147, 556–564.
Jahnke, L., and Klein, H.P. (1983)J. Bacteriol. 155, 488–492.
Popjak, G. (1969)Methods in Enzymology 15, 393–454.
Van Tamelen, E.E., and Curphey, T.J. (1962)Tetrahedron Letters 3, 121–124.
Andreasen, A.A., and Stier, T.J.B. (1953) (a)J. Cell. Comp. Physiol. 41, 23–26; (b)J. Cell. Comp. Physiol. 43, 271–282.
Pinto, W.J., Lozano, R., and Nes, W.R. (1985)Biochim. Biophys. Acta 836, 89–95.
Karst, F., and Lacroute, F. (1973)Biochem. Biophys. Res. Commun. 52, 741–747.
Rodriguez, R.J., Low, C., Bottema, C.D.K., and Parks, L. (1985)Biochim. Biophys. Acta 837, 336–343.
Lala, A.K., Lin, H.K., and Bloch, K. (1978)Bioorg. Chem. 7, 437–440.
Dahl, J., Dahl, C., and Bloch, K. (1980)Biochemistry 19, 1462–1467.
Field, R.B., and Holmlund, C.E. (1976)Biochim. Biophys. Acta 180, 465–471.
Faust, J.R., Goldstein, J.L., and Brown, M.S. (1979)Proc. Natl. Acad. Sci. USA 76, 5018–5022.
Caras, I.W., Friedlander, E.J., and Bloch, K. (1980)J. Biol. Chem. 255, 3575–3580.
Author information
Authors and Affiliations
About this article
Cite this article
M'Baya, B., Fegueur, M., Servouse, M. et al. Regulation of squalene synthetase and squalene epoxidase activities insaccharomyces cerevisiae . Lipids 24, 1020–1023 (1989). https://doi.org/10.1007/BF02544072
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF02544072