Abstract
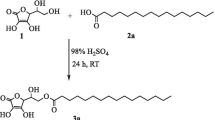
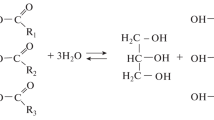
A novel process for the industrial production of hydroxylated fatty acids involves epoxidation of plant oils and their derivatives, followed by catalytic epoxy ring opening in the presence of water or other hydrogen donors, such as alcohols, diols, and amines. Depending on the starting material, epoxidation followed by opening of the oxirane ring leads to fatty acids that contain vicinal diol groups or to other substituted hydroxylated fatty acid derivatives. As an example for the preparation of a substituted hydroxylated fatty acid derivative, the reaction of epoxidized rapeseed oil with monobutylamine as hydrogen donor is described. Apart from the intended formation of hydroxyl groups with vicinal aminoalkyl groups, partial aminolysis of the ester compound was also observed. Another example describes the reaction of epoxidized rapeseed oil with different molar proportions of 1,4-butanediol as hydrogen donor. Depending on the molar proportion of the hydrogen donor, interesterification, or intermolecular ether formation were observed as side reactions. The properties of various technical hydroxylated fatty acids and their derivatives, prepared according to this novel process, are given, and potential applications of these products are suggested.
Similar content being viewed by others
References
Vignolo, R., and F. Naughton,INFORM 2:692 (1991).
Bromsted, J.L.,J. Am. Chem. Soc. 51:428 (1929).
Luong, T.M., H. Schriftman and D. Swern, Ibid.:316 (1967).
Gruber, B., R. Höfer, H. Kluth and A. Meffert,Fat Sci. Technol. 89:147 (1987).
Swern, D., and J.T. Scanlan, U.S. Patent 2,443,280 (1948).
Morrisroe, J.J., and T. Banigan, U.S. Patent 4,101,589 (1978).
Käbisch, G., European Patent EP 002594031 (1980).
Lichtenstein, H.J.,Trans. Faraday Soc. 44:905 (1948).
Jpn. Kokai Tokkyo Koho JP 63-13993.
Shani, A.,Ind. Eng. Chem. Prod. Res. Dev. 22:121 (1983).
Hellbardt, S., K. Schlandt and W.H. Zech, German Patent DE 4125031 (1993).
Hellbardt, S., K. Schlandt and W.H. Zech, German Patent DE 4203077 A1 (1993).
Hellbardt, S., K. Schlandt and W.H. Zech, European Patent Application EP 92250277.8 (1993).
Hellbardt, S., K. Schlandt and W.H. Zech, German Patent Application P 4332292.1 (1993).
Frische, R., K. Wollmann, J. Volkheimer, H. Schomann, J. Schneider, R. Groß-Lannet, A. Ach and B. Best, German Patent DE 4019087 A1 (1991).
Frische, R., and J. Volkheimer, German Patent DE 4024364 A1 (1991).
Frische, R., and J. Volkheimer, German Patent DE 4024365 A1 (1991).
Burg, D., R. Kleiman and S. Erhan, PCT/US92/01263 (1992).
Klauck, W., and P. Daute, PCT/EP92/01308 (1992).
Lincoln, R.M., and J.A. Meyers, U.S. Patent 3,607,778 (1971).
Meffert, A., German Patent DE 3318596 A1 (1984).
Anzinger, H., K. Worschech and B. Wegemund, German Patent DE 3442176 A1 (1986).
Anzinger, H., H. Fischer, H.H. Friese, A. Meffert and H. Schulz, German Patent DE 3507505 A1 (1986).
Rutzen, H., and G. Stoll, German Patent DE 3719790 A1 (1987).
Klauck, W., and P. Daute, German Patent DE 4120432 A1 (1991).
Borggrefe, G., German Patent DE 3203491 C2 (1990).
Meffert, A., and H.C. Wilk, German Patent DE 3638918 A1 (1988).
DIN Deutsches Institut für Normung e.V., Beuth Verlag GmbH, Berlin, Germany, 1985, DIN 53402.
German Society for Fat Science (DGF),German Standard Methods for the Analysis of Fats and Other Lipids, Wissenschaftliche Verlagsgesellschaft mbH, Stuttgart, Germany, 1994, DGF Standard Method C-V 17 a.
Deistra, R., and E. Damen,Anal. Chem. Acta 31:38 (1964).
DIN Deutsches Institut für Normung e.V., Beuth Verlag GmbH, Berlin, Germany, 1985, DIN 16945.
Ingold, C.K.,Structure and Mechanism in Organic Chemistry, Cornell University Press, New York, 1953.
Weber, N., K. Vosmann, E. Fehling, K.D. Mukherjee and D. Bergenstahl,J. Am. Oil Chem. Soc. 72:361 (1995).
Fehling, E., Ibid.:355 (1995).
Author information
Authors and Affiliations
About this article
Cite this article
Dahlke, B., Hellbardt, S., Paetow, M. et al. Polyhydroxy fatty acids and their derivatives from plant oils. J Am Oil Chem Soc 72, 349–353 (1995). https://doi.org/10.1007/BF02541095
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF02541095