Abstract
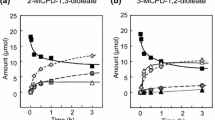
Cholesterol 5α,6α-epoxide (α-epoxide) and cholesterol 5β,6β-epoxide (β-epoxide) were individually suspended in simulated gastric juice (pH 1.2) at 37 C, and their reaction was followed by gradient high performance liquid chromatography (HPLC) with flame ionization (FID) detection. Both epoxides reacted rapidly in the aqueous acid medium. The α-epoxide formed 6β-chlorocholestane-3β,5α-diol (α-chlorohydrin) and 5α-cholestane-3β,5,6β-triol (triol), while the β-epoxide formed 5α-chlorocholestane-3β,6β-diol (β-chlorohydrin) and triol. The isomeric chlorohydrins reacted further to form the triol. In mildly alkaline aqueous medium, each chlorohydrin reverted to the epoxide from which it was formed. The data suggest that both epoxides, which have been reported to have adverse health effects in animals, would be largely hydrolyzed in the stomach and to the triol, which also has been reported to have biological activity. The data furher suggest that residual chlorohydrins surviving stomach residence can be expected to revert to epoxide in the more alkaline intestinal environment.
Similar content being viewed by others
Abbreviations
- α-Chlorohydrin:
-
6β-chlorocholestane-3β,5α-diol
- α-epoxide:
-
cholesterol 5α,6α-epoxide
- β-chlorohydrin:
-
5α-chlorocholestane-3β,6β-diol
- β-epoxide:
-
cholesterol 5β,6β-epoxide
- triol:
-
5α-cholestane-3β,5,6β-triol
- FID:
-
flame ionization detector
- GC:
-
gas chromatography
- HPLC:
-
high performance liquid chromatography
- TLC:
-
thin layer chromatography
References
Smith, L.L. (1981)Cholesterol Autoxidation, Plenum Press, New York.
Maerker, G. (1987)J. Am. Oil Chem. Soc. 64, 388–392.
Addis, P.B. Csallany, A.S., and Kindom, S.E. (1983) inACS Symposium Series No. 234 (Finley, J.W., and Schwass, D.E., eds.) pp. 85–98, American Chemical Society, Washington, D.C.
Imai, H., Werthessen, N.T., Subramanyam, V., LeQuesne, P., Soloway, A.H., and Kanisawa, M. (1980)Science 207, 651–653.
Kelsey, M.I., and Pienta, R.J. (1979)Cancer Lett. 6, 143–149.
Kelsey, M.I., and Pienta, R.J. (1981)Toxicol. Lett. 9, 177–182.
Hill, J.C., Peng, S.-K., Morin, R.J., and Taylor, C.B. (1984)Exp. Mol. Pathol. 41, 249–257.
Peng, S.-K., and Taylor, C.B. (1984) inWorld Review of Nutrition and Dietetics (Bourne, G.H., ed.) Vol. 44, pp. 117–154, S. Karger, Basel.
Peng, S.-K., Taylor, C.B., Hill, J.C., and Morin, R.J. (1985)Atherosclerosis 54, 121–133.
Peng, S.-K., Morin, R.J., Tham, P., and Taylor, C.B. (1985)Artery, 13, 144–164.
Sevanian, A., and Peterson, A.R. (1984)Proc. Natl. Acad. Sci. USA 81, 4198–4202.
Smith, L.L., Smart, V.B., and Made Gowda, N.M. (1986)Mutat. Res. 161, 39–48.
Raaphorst, G.P., Azzam, E.I., Langlois, R., and VanLier, J.E. (1987)Biochem. Pharmacol. 36, 2369–2372.
Maerker, G., and Bunick, F.J. (1986)J. Am. Oil Chem. Soc. 63, 771–777.
Park, S.W., and Addis, P.B. (1986)J. Agric. Food Chem. 34, 653–659.
Bascoul, J., Domerque, N., Ole, M., and Crastes de Paulet, A. (1986)Lipids 21, 383–387.
Finocchiaro, E.T., and Richardson, T. (1983)J. Food Prot. 46, 917–925.
Tsai, L.-S., and Hudson, C.A. (1985)J. Food Sci. 50, 229–231, 237.
Missler, S.R., Wasilchuk, B.A., and Merritt, C., Jr. (1985)J. Food Sci. 50, 595–598, 646.
Fischer, K.-H., Laskawy, G., and Grosch, W. (1985)Z. Lebensm. Unters. Forsch. 181, 14–19.
Sugino, K., Terao, J., Murakami, H., and Matsushita, S. (1986)J. Agric. Food. Chem. 34, 36–39.
Csiky, I. (1982)J. Chromatog. 241, 381–389.
Luby, J.M., Gray, J.I., Harte, B.R., and Ryan, T.C. (1986)J. Food Sci. 51, 904–907, 923.
Finocchiaro, E.T., Lee, K., and Richardson, T. (1984)J. Am. Oil Chem. Soc. 61, 877–883.
Lee, K., Herian, A.M., and Higley, N.A. (1985)J. Food Prot. 48, 158–161.
Higley, N.A., Taylor, S.L., Herian, A.M., and Lee, K. (1986)Meat Sci. 16, 175–188.
Barton, D.H.R., and Miller, E. (1950)J. Am. Chem. Soc. 72, 370–374.
Rivett, D.E.A., and Wallis, E.S. (1950)J. Org. Chem. 15, 35–41.
Windaus, A. (1921) Hoppe-Seyler'sZ. Physiol. Chem. 117, 146–155.
Baxter, R.A., and Spring, F.S. (1943)J. Chem. Soc., 613–615.
Plattner, P.A., and Lang, W. (1944)Helv. Chim. Acta. 27, 1872–1875.
Fieser, L.F., and Rajagopalan, S. (1949)J. Am. Chem. Soc. 71, 3938–3941.
Davis, M., and Petrow, V. (1949)J. Chem. Soc., 2536–2539.
Chicoye, E., Pourie, W.D., and Fennema, O. (1968)Lipids 3, 335–339.
Maerker, G., Nungesser, E.H., and Zulak, I.M. (1988)J. Agric. Food Chem. 15, 61–63.
Leach, A.A. (1961) inBiochemists' Handbook (Long, C., ed.) pp. 911–914, D. Van Nostrand Company, Inc., Princeton, NJ.
Hawk, P.B., Oser, B.L., and Summerson, W.H. (1954) inPractical Physiological Chemistry, 13th edn., p. 368, McGraw Hill Book Co., New York.
Author information
Authors and Affiliations
About this article
Cite this article
Maerker, G., Nungesser, E.H. & Bunick, F.J. Reaction of cholesterol 5,6-epoxides with simulated gastric juice. Lipids 23, 761–765 (1988). https://doi.org/10.1007/BF02536218
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF02536218