Abstract
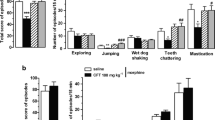
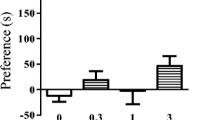
The relative involvement of μ- and δ-opioid receptors in the mediation of butorphanol-, as compared to morphine-, dependence was examined with the use of highly selective antagonists at μ- and δ-opioid receptors. Extracellular fluid levels of glutamate (Glu) and aspartate (Asp) were measured within the pontine locus coeruleus following precipitation of withdrawal from dependence on either butorphanol or morphine in conscious Sprague-Dawley rats. Dependence was induced by intracerebroventricular (i.c.v.) infusion of butorphanol (26 nmol/μl/h), morphine (26 nmol/μl/h) or saline vehicle (1 μl/h) for 3 days by means of an osmotic minipump. Microdialysis probes (2 mm tip) were inserted into the locus coeruleus 24 h before precipitation of withdrawal by i.c.v. injection of either the μ-opioid receptor antagonist,d-Pen-Cys-Tyr-d-Trp-Orn-Thr-Pen-Thr-NH2 (CTOP; 4.8 nmol/5 μl or 48 nmol/5 μl), or the δ-opioid receptor antagonist, naltrindole (17-cyclopropylmethyl-6,7-dehydro-4,5-epoxy-3,14-dihydroxy-6,7,2′3′-indolmorphinan hydrochloride; 48 nmol/5 μl or 100 nmol/5 μl). Baseline levels of Glu ranged from 9.59±1.27 to 12.84 ±3.01 μM in the various treatment groups. Level of Asp were similar. Precipitation of withdrawal by CTOP elicited significant increases of Glu and Asp in both morphine- and butorphanol-dependent rats. Maximal increases in Glu of 425% and 258% above baseline levels were elicited in the first 15 min microdialysis sample following i.c.v. injection of CTOP in morphine- and butorphanol-dependent rats, respectively. Behavioral signs of withdrawal were greater in morphine than butorphanol-dependent groups. The i.c.v. treatment with naltrindole elicited increases in Glu and Asp that were similar, although less marked, than those precipitated by CTOP treatment. Administration of naltrindole produced equivalent signs of withdrawal in both morphine- and butorphanol-dependent rats. Withdrawal from dependence on both morphine and butorphanol is characterized by elevations in coerulear levels of excitatory amino acids. Responses elicited following the use of selective μ- and δ-opioid receptor antagonists to precipitate withdrawal suggest that the role played by these receptors in mediation of the signs and symptoms of withdrawal do not differ greatly between butorphanol- and morphine-dependent rats.
Similar content being viewed by others
References
Abdelhamid, E. E., Sultana, M., Portoghese, P. S., and Takemori, A. E. 1991. Selective blockage of delta opioid receptors prevents the development of morphine tolerance and dependence in mice. J. Pharmacol. Exp. Ther. 258:299–301.
Heel, R. C., Brogden, R. N., Speight, T. M., and Avery, G. S. 1978. Butorphanol: A review of its pharmacological properties and therapeutic efficacy.Drugs 16:473–505.
Horan, P., and Ho, I. K. 1989. Comparative pharmacological and biochemical studies between butorphanol and morphine. Pharmacol. Biochem. Behav. 34:847–854.
Horan, P., and Ho, I. K. 1991. The physical dependence liability of butorphanol: a comparative study with morphine. Eur. J. Pharmacol. 203:397–391.
Jaw, S. P., Hoskins, B., and Ho, I. K. 1993. Involvement of δ-opioid receptors in physical dependence on butorphanol. Eur. J. Pharmacol. 240:67–72.
Jaw, S. P., Hopkins, B., and Ho, I. K. 1993. Opioid antagonists and butorphanol dependence. Pharmacol. Biochem. Behav. 44: 497–500.
Martin, W. R., Eades, C. G., Thompson, J. A., Huppler, R. E., and Gilbert, P. E. 1976. The effects of morphine- and nalorphine-like drugs in the nondependent and morphine-dependent chronic spinal dog. J. Pharmacol. Exp. Ther. 197:517–532.
Miyamoto, Y., Portoghese, P. S., and Takemori, A. E. 1993. Involvement of delta2 opioid receptors in the development of morphine dependence in mice. J. Pharmacol. Exp. Ther. 264:1141–1145.
Monkovic, I., Conway, T. T., Wong, H., Perron, Y. G., Pachter, I. J., and Beieau, B. (1973). Total synthesis and pharmacological activities on N-substituted 3,14-dihydroxymorphinans, J. Am. Chem. Soc. 95:7910–7911.
Oh, K. W., Makimura, M., Jaw, S. P., Hoskins, B., and Ho, I. K. 1992. Effects of β-funaltrexamine on butorphanol dependence. Pharmacol. Biochem. Behav. 42:29–34.
Picker, M. J., Negus, S. S., and Craft, R. M. 1990. Butorphanol’s efficacy at mu and kappa opoid receptors: inferences based on the schedule-controlled behavior of nontolerant and morphine-tolerant rats and on the responding of rats under a drug discrimination procedure. Pharmacol. Biochem. Behav. 36:563–568.
Pircio, A. W., Gylys, J. A., Cananagh, R. L., Buynishi, J. P., and Bierwagen, M. E. 1976. The pharmacology of butorphanol, a 3,14-dihydroxymorphinan narcotic antagonist analgesic. Arch. Int. Pharmacodyn. 220:231–257.
Gulya, K., Krivan, M., Nyolczas, N., Sarnyai, Z., and Kovacs, G. L. 1988. Central effects of the potent and highly selective μ opioid antagonistd-Phe-Cys-Tyr-D-Trp-Orn-Thr-Pen-Thr-NH2 (CTOP) in mice. Eur. J. Pharmacol. 150:355–360.
Aghajanian, G. K. 1978. Tolerance of locus neurons to morphine and suppression of withdrawal response by clonidine. Nature 276: 186–188.
Feng, Y. Z., Zhang, T., Rockhold, R. W., and Ho, I. K. 1995. Increased locus coeruleus glutamate levels are associated with naloxone-precipitated withdrawal from butorphanol in the rat, Neurochem. Res. 20:745–751.
Redmond, D. E., Jr., and Krystal, J. H. 1984. Multiple mechanisms of withdrawal from opioid drugs. Annu. Rev. Neurosci. 7:443–478.
Foote, S. L., Bloom, F. E., and Aston-Jones, G. (1983). Nucleus locus coeruleus: new evidence of anatomical and physiological specificity. Physiol. Rev. 63:844–914.
Rasmussen, K., and Aghajanian, G. K. (1989). Withdrawal-induced activation of locus coeruleus neurons in opiate-dependent rats: attenuation by lesions of the nucleus paragigantocellularis. Brain Res. 505:346–450.
Maldonado R., Stinus, L., Gold, L. H., and Koob, G. F. (1992). Role of different brain structures in the expression of the physical morphine withdrawal syndrome. J. Pharmacol. Exp. Ther. 261: 669–677,
Aghajanian, G. K., Kogan, J. H., and Moghaddam, B. 1994. Opiate withdrawal increase glutamate and aspartate effux in the locus coeruleus: an in vivo microdialysis study. Brain Res. 636:126–130.
Hong, M., Milne, B., and Jhamandas, K. 1993. Evidence for the involvement of excitatory amino acid pathways in the development of precipitated withdrawal from acute and chronic morphine: an in vivo voltammetric study in the rat locus coeruleus. Brain Res. 623:131–141.
Zhang, T., Feng, Y. Z., Rockhold, R. W., and Ho, I. K. 1994. Naloxone-precipitated morphine withdrawal increases pontine glutamate levels in the rat. Life Sci. 55:PL25–31.
Rasmussen, K., Beitner-Jonson, D. B., Krystal, J. H., Aghajanian, G. K., and Nestler, E. J. 1990. Opiate withdrawal and the rat locus coeruleus: behavioral, electrophysiological, and biochemical correlates. J. Neurosci. 10:2308–2317.
Rasmussen, K., Krystal, J. H., and Aghajanian, G. K. 1991. Excitatory amino acids and morphine withdrawal: differential effects of central and peripheral kynurenic acid administration. Psychopharmacology. 105:508–512.
Tanganelli, S., Antonelli, T., Morari M. Bianchi, C., and Beani, L. 1991. Glutamate antagonists prevent morphine withdrawal in mice and guinea pigs. Neurosci. Lett. 122:270–272.
Trujillo, K. A., and Akil, H. 1991. Inhibition of morphine tolerance and dependence by the NMDA receptor antagonist MK-801, Science 251:85–87.
Robers, H., Hyes, A. G., Birch, P. J., Traynor, J. R., and Lawrence, A. J. 1990. The selectivity of the opioid antagonist, naltrindole, for δ-opioid receptors. J. Pharm. Pharmacol. 42:358–359.
Paxinos, G., and Watson, C. 1986. The Rat Brain in Stereotaxic Coordinates, 2nd edition, Academic Press, Orlando, Florida.
Feng, Y. Z., Narita, M., Tseng, Y. T., Hoskins, B., and Ho, I. K. 1994. Crosstolerance between butorphanol and morphine in rats. Pharmacol. Biochem. Behavior. 49:657–661.
Ellison, D. W., Beal, M. F., and Martin, J. B. 1987. Amino acid neurotransmitters in postmortem human brain analyzed by high performance liquid chromatography with electrochemical detection. J. Neurosci. Methods. 19:305–315.
McCarthy, P. S., Metacalf, G., and Howe, S. J. 1982. Continous infusion in rats as a method of assessing morphine-like physical dependence for opiates. Pharmacol. Biochem. Behav. 16:725–729.
McCarthy, P. S., and Howlett, G. J. 1984. Physical dependence induced by opiate partial agonists in the rat. Neuropeptides 5:11–14.
Brown, G. R. 1985. Stadol dependence: Another case. J. Am. Med. Assoc. 254:910.
Evans, W. S., Bowen, J. N., Giordano, F.L., and Clark, B. 1985. A case of stadol dependence. J. Am. Med. Assoc. 253:2191–2192.
Hoover, R. C., and Williams, R. B. 1985. Survey of butorphanol and nalbuphine diversion in U.S. hospitals. Am. J. Hosp. Pharm. 42:1111–1113.
Jasinski, D. R., Preston, K. L. and Testa, M. 1988. Abuse potential evaluation of transnasally given butorphanol in humans. The Pharmacologist 30:A196.
Smith, S. G., and Davis, W. M. 1984. Nonmedical use of butorphanol and diphenhydramine. J. Am. Med. Assoc. 252:1010.
Rasmussen, K. 1991. Afferent effects on locus coeruleus in opiate withdrawal, In Progress in Brain Research (Barnes, C. D., and Pompeiano, O., eds.), 88:207–216. Elsevier Science Publishers B. V., Amsterdam, Netherlands.
Feng, Y., Zhang, T., Rockhold, R. W. and Ho, I. K. 1994. Opioid receptor subtypes modulate pontine excitatory amino acid (EAA) levels differently in butorphanol and morphine dependence. Soc. Neurosci. Abst. 20:1167.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Feng, Y.Z., Zhang, T., Tokuyama, S. et al. μ- and δ-opioid receptor antagonists precipitate similar withdrawal phenomena in butorphanol and morphine dependence. Neurochem Res 21, 63–71 (1996). https://doi.org/10.1007/BF02527673
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF02527673