Abstract
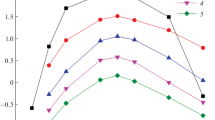
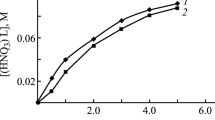
The nitrate salt solution of the secondary amine Amberlite LA-1 in organic solvents extracts uranium(IV) from aqueous nitric acid solutions. The distribution ratio of uranium(IV) reaches a maximum at an equilibrium nitric acid concentration of 8.5M in the aqueous phase. Addition of n-octanol to the organic phase decreases, and the addition of nitrate to the aqueous phase increases the uranium(IV) distribution ratio. The extraction of uranium(IV) is fast and the equilibrium distribution is reached within less than one minute. At low uranium(IV) concentrations (<6·10−3 M) the distribution ratio is independent of the uranium(IV) concentration. At high uranium(IV) loadings of the organic phase an extrapolation gives a mole ratio of amine: uranium(IV)=2∶1. A double logarithmic plot of the dependence of the uranium(IV) distribution ratio vs. the LA-1 concentration in the organic phase gives a curve with a slope of two when polar diluents for LA-1 are used. This slope of two and the shapes of the absorption spectra of the organic phase from 400 to 700 nm make it very probable that uranium(IV) exists in the organic phase as a hexanitrato complex.
Similar content being viewed by others
Literatur
G. Koch, E. Schwind,J. Inorg. Nucl. Chem., 28 (1966) 871.
W. E. Keder, A. S. Wilson,J. Inorg. Nucl. Chem., 18 (1961) 259.
C. F. Coleman, C. A. Blake, K. W. Brown,Talanta, 9 (1962) 297.
A. S. Kertes, I. T. Platzner,J. Inorg. Nucl. Chem., 24 (1962) 1419.
Broschüre IE-41-58; Rohm und Haas Co., Washington Square, Philadelphia 5, Pa., USA.
Ind. Eng. Chem., 53 (1961) 69A.
C. Boirie,Bull. Soc. Chim. France, (1958) 980.
E. R. Schmid,Mikrochim. Acta, (1970) 301.
Y. Umezaki,Bull. Chem. Soc. Japan, 36 (1963) 769.
J. H. Yoe, F. Will, R. A. Black,Anal. Chem., 25 (1953) 1200.
E. R. Schmid, E. Jünger,Z. Anal. Chem., 257 (1971) 112.
J. Bizot, B. Tremillon,Bull. Soc. Chim. France, (1959) 122.
C. F. Coleman, K. B. Brown, J. G. Moore, D. J. Crouse,Ind. Eng. Chem., 50 (1958) 1756.
M. Taube,J. Inorg. Nucl. Chem., 12 (1960) 134.
W. E. Keder, J. L. Ryan, A. S. Wilson,J. Inorg. Nucl. Chem., 20 (1961) 131.
U. Bertocci, G. Rolandi,J. Inorg. Nucl. Chem., 23 (1961) 323.
J. M. P. Verstegen,J. Inorg. Nucl. Chem., 26 (1964) 1085.
G. Scibona, B. Scuppa, M. Zifferero,Energia Nucl. (Milan), 12 (1965) 85.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Schmid, E.R., Jünger, E. Die Verteilung von Uran(IV) zwischen wässriger Salpetersäure und organischen Lösungen des Nitratsalzes des sekundären Amins Amberlite LA-1. J. Radioanal. Chem. 11, 35–47 (1972). https://doi.org/10.1007/BF02518616
Received:
Issue Date:
DOI: https://doi.org/10.1007/BF02518616