Abstract
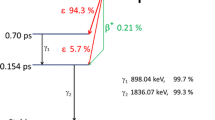
A method is described for the radioanalytical determination of traces of chlorine in aqueous solutions without radiochemical separation or purification. Using a gamma-spectrometer with monocrystal scintillator, the sensitivity of the analysis is about 1·10−8 g of chlorine/ml, the time of analysis being 15 minutes. For the selective determination of chlorine in aqueous solutions containing a large amount of impurities, a bicrystal scintillation sum-coincidence spectrometer was employed with 120×100 mm NaI(Tl) crystals and thus the38Cl cascade radiation could be used. Application of the sum-coincidence spectrometer allowed a reliable determination of 1·10−7 g of Cl/ml against a background of 1·10−5 g of Na/ml.
Similar content being viewed by others
References
V. P. Sokolov,Zh. Prikl. Khim., 37 (1964) 187.
V. P. Sokolov,Zavodsk. Lab., 3 (1962) 285.
A. G. Souliotis, A. P. Grimanis, N. A. Tsanos,Talanta, 13 (1966) 158.
H. R. Lukens, F. M. Graber, D. M. Settle,Trans. Amer. Nucl. Soc., 9 (1966) 88.
V. A. Blinov, V. N. Dmitriyev, M. I. Kuznetsov,At. Energ. (USSR), 19 (1965) 342.
E. T. Bramlitt,Anal. Chem., 38 (1966) 1669.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Yazikov, I.F., Rodin, N.N., Dembrovsky, M.A. et al. Applications of gamma-spectrometry in determining chlorine in aqueous solutions. J. Radioanal. Chem. 3, 11–16 (1969). https://doi.org/10.1007/BF02513992
Received:
Revised:
Issue Date:
DOI: https://doi.org/10.1007/BF02513992