Abstract
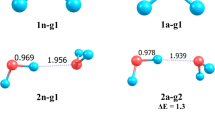
Structural changes that occur in cyclic and chain-like water pentamers and hexamers when an electron is added were analyzed at the unrestricted Hartree—Fock level. The vertical and adiabatic electron affinity of the oligomers, the energies of vertical detachment of an electron from the anions (VDE), and the stability of the anions against dissociation into individual water molecules and a free electron were estimated taking into account the second order perturbation theory (MP2) corrections to the energy. All water anions considered are stable against dissociation, but theirVDE values are negative, and only the chain-like hexamer anion has a value ofVDE close to zero, which means metastability of the anion. The energy of attachment of an electron to the oligomers is lower in the case of chain-like structures. The process is accompanied by structure relaxation, which is more substantial for cycles, especially for the cyclic hexamer. In the chain-like anions, the excess electron density is localized on the hydrogen nuclei of that terminal water molecule that acts as an acceptor of the H-bond proton, while in the cyclic anions it is distributed on the orbitals of those free hydrogen atoms that significantly approach each other due to structural relaxation.
Similar content being viewed by others
References
Yu. V. Novakovskaya and N. F. Stepanov,Izv. Akad. Nauk, Ser. Khim., 1997, 41 [Russ. Chem. Bull., 1997,46, 36 (Engl. Transl.)].
M. W. Schmidt, K. K. Baldridge, J. A. Boatz, S. T. Elbert, M. S. Gordon, J. H. Jensen, S. Koseki, N. Matsunaga, K. A. Nguyen, S. J. Su, T. L. Windus, M. Dupuis, and J. A. Montgomery,J. Comput. Chem., 1993,14, 1347.
M. D. Newton,J. Phys. Chem., 1975,79, 2795.
J. O. Noel and K. Morokuma,J. Phys. Chem., 1977,81, 2295.
B. K. Rao and N. R. Kestner,J. Chem. Phys., 1984,80, 1587.
N. R. Kestner and J. Jortner,J. Phys. Chem., 1984,88, 3813.
H. Haberland, H. Langosch, H. -G. Schindler, and D. R. Worsnop,J. Phys. Chem., 1984,88, 3903.
H. Haberland, C. Ludewigt, H. -G. Schindler, and D. R. Worsnop,J. Chem. Phys., 1984,81, 3742.
J. V. Coe, G. H. Lee, J. G. Eaton, S. T. Arnold, H. W. Sarkas, K. H. Bowen, C. Ludewigt, H. Haberland, and D. R. Worsnop,J. Chem. Phys., 1990,92, 3980.
Author information
Authors and Affiliations
Additional information
Translated fromIzvestiya Akademii Nauk, Seriya Khimicheskaya, No. 1, pp. 47–53, January 1997
Rights and permissions
About this article
Cite this article
Novakovskaya, Y.V., Stepanov, N.F. The possibility of the existence of (H2O) n − anions withn=5, 6. Russ Chem Bull 46, 42–48 (1997). https://doi.org/10.1007/BF02495344
Received:
Issue Date:
DOI: https://doi.org/10.1007/BF02495344