Abstract
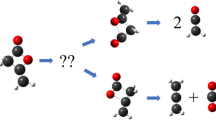
The products and kinetics of the thermal decomposition of dimethyldioxirane (DMDO) were studied. The reaction proceedsvia three parallel pathways: isomerization to methyl acetate, oxygen atom insertion into the C−H bond of a solvent molecule (acetone), and the solvent-induced homolysis of the O−O bond in the DMDO molecule. The contribution of the latter reaction channel isca. 23% at 56°C. The overall kinetic parameters of DMDO thermolysis in oxygen atmosphere were determined. The free radical-induced DMDO decomposition occurs in an inert atmosphere. The formal kinetics of this reaction was investigated. The mechanism of the DMDO thermolysis is discussed.
Similar content being viewed by others
References
W. Adam, R. Curci, and J. O. Edwards,Acc. Chem. Res., 1989,22, 205.
R. W. MurrayChem. Rev., 1989,89, 1187.
W. Adam and L. Hadjiarapoglou,Top. Curr. Chem., 1993,164, 45.
R. Curci, A. Dinoi, and M. F. Rubino,Pure and Appl. Chem., 1995,67, 811.
R. W. Murray and H. Gu,J. Org. Chem., 1995,60, 5673.
R. W. Murray and H. Gu,J. Chem. Soc., Perkin Trans. 2, 1994, 451.
R. W. Murray, R. Jeyaraman, and L. Mohan,J. Am. Chem. Soc., 1986,108, 2470.
R. Mello, M. Fiorentino, C. Fusco, and R. Curci,J. Am. Chem. Soc., 1989,111, 6749.
W. Adam, R. Curci, M. E. G. Nunez, and R. Mello,J. Am. Chem. Soc., 1991,113, 7654.
M. Singh and R. W. Murray,J. Org. Chem., 1992,57, 4263.
F. Minisci, L. Zhao, F. Fontana, and A. Bravo,Tetrahedron Lett., 1995,36, 1697.
F. Minisci, L. Zhao, F. Fontana, and A. Bravo,Tetrahedron Lett., 1995,36, 1895.
A. Bravo, F. Fontana, G. Fronza, A. Mele, and F. Minisci,J. Chem. Soc., Chem. Commun., 1995, 1573.
R. Curci, A. Dinoi, C. Fusco, and M. A. Lillo,Tetrahedron Lett., 1996,37, 249.
A. Dinoi, R. Curci, P. Carloni, E. Damiani, P. Stipa, and L. Greci,Eur. J. Org. Chem., 1998, 871.
W. Adam, R. Curci, L. D'Accolti, A. Dinoi, C. Fusco, F. Gasparrini, R. Kluge, R. Paredes, M. Schulz, A. K. Smerz, L. Angela Veloza, S. Weinkotz, and R. Winde,Chem. Eur. J., 1997,3, 105.
P. A. Simakov, S. -Y. Choi, and M. Newcomb,Tetrahedron Lett., 1998,39, 8187.
G. Asensio, R. Mello, M. E. Gonzalez-Nunez, C. Boix, and J. Royo,Tetrahedron Lett., 1997,38, 2373.
A. Bravo, F. Fontana, G. Fronza, F. Minisci, and L. Zhao,J. Org. Chem., 1998,63, 254.
D. V. Kazakov, N. N. Kabal'nova, S. L. Khursan, and V. V. Shereshovets,Izv. Akad. Nauk, Ser. Khim., 1997, 694 [Russ. Chem. Bull., 1997,46, 663 (Engl. Transl.)].
L. A. Hull and L. Budhai,Tetrahedron Lett., 1993,34, 5039.
S. A. Grabowski, D. V. Kazakov, N. N. Kabalnova, S. L. Khursan, and V. V. Shereshovets,React. Kinet. Catal. Lett., 1997,62, 179.
S. A. Grabowski, N. N. Kabal'nova, S. L. Khursan, and V. V. Shereshovets,Izv. Akad. Nauk, Ser. Khim., 1998, 1321 [Russ. Chem. Bull., 1998,47, 1284 (Engl. Transl.)].
D. V. Kazakov, D. R. Khusnullina, N. N. Kabal'nova, S. L. Khursan, and V. V. Shereshovets,Izv. Akad. Nauk, Ser. Khim., 1997, 1785 [Russ. Chem. Bull., 1997,46, 1690 (Engl. Transl.)].
D. R. Khusnullina, D. V. Kazakov, N. N. Kabal'nova, S. L. Khursan, and V. V. Shereshovets,Kinet. Katal., 1998,39, 8 [Kinet. Catal., 1998,39 (Engl. Transl.)].
R. W. Murray and R. Jeyaraman,J. Org. Chem., 1985,50, 2847.
W. Adam, J. Bialas, and L. Hadjiarapoglou,Chem. Ber., 1991,124, 2377.
V. B. Kogan, V. M. Fridman, and V. V. Kafarov,Spravochnik po rastvorimosti [Handbook on Solubility], Izd-vo Akad. Nauk SSSR, Moscow, 1961,1, book 1 (in Russian).
M. Ferrer, M. Gibert, F. Sanchez-Baeza, and A. Messeguer,Tetrahedron Lett., 1996,37, 3585.
D. Cremer, E. Kraka, and P. G. Szalay,Chem. Phys. Lett., 1998,292, 97.
P. Neta, R. E. Huie, and A. R. Ross,J. Phys. Chem. Ref. Data, 1990,19, 413.
E. T. Denisov and V. V. Azatyan,Ingibirovanie tsepnykh reaktsii [The Inhibition of Chain Reactions], Izd-vo IKhFCh RAN, Chernogolovka, 1997 (in Russian).
G. V. Shustov and A. Rauk,J. Org. Chem., 1998,63, 5413.
G. A. Russell,J. Am. Chem. Soc., 1957,79, 3871.
J. A. Howard,Advances in Free Radical Chemistry, 1972,4, 49.
D. G. Hendry, T. Mill, L. Piszkiewicz, J. A. Howard, and H. K. Eigenmann,J. Phys. Chem. Ref. Data, 1974,3, 937.
H. Paul, R. D. Small, and J. C. Scaiano,J. Am. Chem. Soc., 1978,100, 4520.
Author information
Authors and Affiliations
Additional information
Dedicated to Professor E. T. Denisov on the occasion of his 70th Birthday.
Published inIzvestiya Akademii Nauk. Seriya Khimicheskaya, No. 8, pp. 1344–1354, August, 2000.
Rights and permissions
About this article
Cite this article
Khursan, S.L., Grabovskii, S.A., Kabal'nova, N.N. et al. The kinetic regularities, products, and mechanism of the thermal decomposition of dimethyldioxirane. The contribution of molecular and radical reaction channels. Russ Chem Bull 49, 1338–1348 (2000). https://doi.org/10.1007/BF02495074
Received:
Revised:
Issue Date:
DOI: https://doi.org/10.1007/BF02495074