Abstract
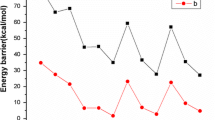
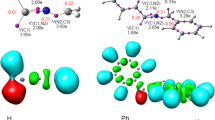
Protonated forms of the molecules of ethylene derivatives with the general formula C2X2Y2 (X=Y=H) (1), F (2), CH3 (3) CH3 (4); X=F, Y=H:cis-(5)trans- (6)) were calculated by theab initio MP2/6-31 G* method with full geometry optimization. The minima and saddle points located on the potential energy surface (PES) of the protonated ethylene molecule correspond to the stationary states and transition states of proton migration, respectively. The stationary states are characterized by a nonclassical geometry of carbocations similar to that of π-complexes, whereas the transition states have a classical structure. Unlike1, the carbocations of molecules2–6 have the classical structure. The saddle points on the PES of the ethylene derivatives correspond to the structures of the π-complex type, which are the transition states of proton migration between the C atoms of the ethylene bond. The barrier to rotation about, the C−C bond depends essentially on the substituent nature.
Similar content being viewed by others
References
M. French and P. Kebarle,Can. J. Chem., 1975,53, 2268.
J. E. Del Bene, M. J. Frisch, K. Raghavachari, and J. A. Pople,J. Phys. Chem., 1982,86, 1529.
E. del Rio, R. Lopez, and L. Sordo,J. Phys. Chem. A., 1998,102, 6831.
Saebo and J. Almlöf,Chem. Phys. Lett., 1989154, 83.
M. J. Frisch, G. W. Trucks, H. B. Schlegel, G. E. Scuseria, M. A. Robb, J. R. Cheeseman, V. G. Zakrzewski, J. A. Montgomery, Jr., R. E. Stratmann, J. C. Burant, S. Dapprich, J. M. Milllam, A. D. Daniels, K. N. Kudin, M. C. Strain, O. Farkas, J. Tomasi, V. Barone, M. Cossi, R. Cammi, B. Mennucci, C. Pomelli, C. Adamo, S. Ceifford, J. Ochterski, G. A. Petersson, P. Y. Ayala, Q. Cui, K. Morokuma, D. K. Malick, A. D. Rabuck, K. Raghavachari, J. B. Foresman, J. Cioslowski, J. V. Ortiz, B. B. Stefanov, G. Liu, D. J. Fox, T. Keith, M. A. Al-Lahm, C. Y. Peng, A. Nanayakkara, C. Gonzalez, M. Challacombe, P. M. W. Gill, B. Johnson, W. Chen, M. W. Wong, J. L. Andres, M. Hea-Gordon, E. S. Replogle, and J. A. Pople,GAUSSIAN-98, Revision A.5, Pittsburgh (PA), 1998.
H. B. Schlegel,J. Comput. Chem., 1982,3, 211.
C. Gonzales and H. B. Schlegel,J. Phys. Chem., 1990,94, 5523.
J. C. Traeger and R. G. McLoughlin,J. Am. Chem. Soc., 1982,103, 3647.
R. L. Stockbauer and J. L. Holmes,J. Am. Chem. Soc., 1982,104, 2337.
Comprehensive Organic Chemistry, Eds. D. Barton and W. D. Ollis, Pergamon Press, Oxford-New York-Toronto-Sydney-Paris-Frankfurt, 1979,1.
J. E. Szulejko and T. B. McMahon,J. Am. Chem. Soc. 1993,115, 7839.
T. P. Ponomareva, V. S. Yushenko, and E. D. Shchukin,Zh. Strukt. Khim., 1995,36, 34 [Russ. J. Struct. Chem., 1995,36 (Engl. Transl.)].
A. V. Fokin and M. A. Landau,Usp. Khim., 1998,67, 28 [Russ. Chem. Rev., 1998,67 (Engl. Transl.)].
Author information
Authors and Affiliations
Additional information
Published inIzvestiya Akademii Nauk. Seriya Khimicheskaya, No. 8, pp. 1333–1337, August, 2000.
Rights and permissions
About this article
Cite this article
Borisov, Y.A. Anab initio quantum-chemical study of proton addition to F-, Ch3-, and CF3-substituted ethylene derivatives. Russ Chem Bull 49, 1327–1331 (2000). https://doi.org/10.1007/BF02495072
Received:
Revised:
Issue Date:
DOI: https://doi.org/10.1007/BF02495072