Abstract
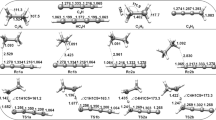
The optimal geometry and energy parameters for five electronic states of the {1O2 (1Δg) + C2H4} system that characterize the elementary reactions of two-step 1,2-addition giving the dioxetane molecule were calculated using various quantum chemical methods (RHF, B3LYP, MPn, n = 2–4, QCISD, and CCSD) and basis sets (from 6-31+G(d,p) to 6-311+G(3df,2p) and pVTZ). The first step of the reaction was found to pass through the ethylene perepoxide intermediate. Considering experimental and published calculated data, the dependence of the results on the calculation procedure was exampled. The higher-level methods (QCISD, CCSD, CASSCF) and the standard methods (DFT, MPn) were found to reliably lead to virtually the same description of the energetics of this two-step reaction corresponding to experimental estimates.
Similar content being viewed by others
References
F. Ogliaro, N. Harris, S. Cohen, et al., Am. Chem. Soc. 122, 8977 (2000).
T. Jovanovic, R. Farid, R. Friesner, and A. McDermott, J. Am. Chem. Soc. 127, 13548 (2005).
K. Yamaguchi, Singlet Oxygen, Ed. by A. Frimer (CRC, Boca Raton, FL, 1985).
K. Yamanuchi, Y. Takahara, and T. Fueno, The Role of Oxygen in Chemistry and Biology, Ed. by W. Ando and Y. Moro-Oka (Elsevier, Amsterdam, 1988), p. 263.
K. Yamanuchi, Y. Takahara, T. Fueno, et al., Medical, Biological and Chemistry Aspects of Free Radicals, Ed. by O. Hayaishi, E. Niki, M. Kondo, and T. Yoshikawa (Elsevier, Amsterdam, 1989), p. 993.
K. Yamanuchi, K. Takada, Y. Otsui, and K. Mizuno, Organic Peroxide, Ed. by W. Ando (Wiley, New York, 1992), p. 2.
T. Omura, Cytochrome P-450, Ed. by Y. Ishimura and Y. Fuijii-Kriyama (Kodansha, Tokyo, 1993).
H. Li, Handbook of Metalloproteins, Ed. by A. Messerschmidt, R. Huber, T. Poulos, and K. Weighardt (Wiley, Oxford, 2001), p. 267.
K. Yamaguchi, T. Fueno, and H. Fukutome, Chem. Phys. Lett. 22, 466 (1973).
J. Cizek and J. Paldus, J. Chem. Phys. 47, 4976 (1967).
H. Fukutome, Prog. Theor. Phys. 47, 1156 (1972).
N. Ostlund, J. Chem. Phys. 47, 2994 (1972).
K. Yamaguchi, J. Mol. Struct. (THECHEM) 103, 101 (1983).
H. Isobe, S. Nishihara, M. Shoji, et al., Int. J. Quantum Chem. 108, 2991 (2008).
Y. Yoshioka, T. Tsunesada, K. Yamaguchi, and I. Saito, Int. J. Quantum Chem. 65, 787 (1997).
K. Yamaguchi, S. Yamanaka, J. Shimada, et al., Int. J. Quantum Chem. 109, 3745 (2009).
T. Saito, S. Nishihara, Y. Kataoka, et al., Chem. Phys. Lett. 483, 168 (2009).
T. Saito, S. Nishihara, Y. Kataoka, et al., J. Phys. Chem. A 114, 7967 (2010).
A. E. Gekhman, A. P. Makarov, V. M. Nekipelov, et al., Izv. Akad. Nauk SSSR, Ser. Khim. 7, 1686 (1985).
N. I. Moiseeva, A. E. Gekhman, S. G. Sakharov, et al., Izv. Akad. Nauk SSSR 10, 2396 (1986).
A. E. Gekhman, N. I. Moiseeva, and I. I. Moiseev, Dokl. Chem. 349, 165 (1996).
A. A. Markov, G. F. Sharifullina, S. P. Dolin, et al., Kinet. Katal. 55, 456 (2014).
D. Bogan, R. Sheison, and F. Williams, J. Am. Chem. Soc. 98, 1034 (1976).
H. Neil and W. Richardson, J. Am. Chem. Soc. 92, 6553 (1970).
D. J. Bogan and F. Williams, Photochem. Photobiol. 30, 3 (1979).
C. Tanaka and J. Tanaka, J. Phys. Chem. A 104, 2078 (2000).
N. B. Filimonova, A. V. Vorob’ev, V. K. Bozhenko, et al., Khim. Fiz. 26, 19 (2010).
N. B. Filimonova, A. V. Vorob’ev, V. K. Bozhenko, et al., Khim. Fiz. 29, 16 (2010).
Author information
Authors and Affiliations
Corresponding author
Additional information
Original Russian Text © S.P. Dolin, N.N. Breslavskaya, A.A. Markov, T.Yu. Mikhailova, N.I. Moiseeva, A.E. Gekhman, 2015, published in Zhurnal Neorganicheskoi Khimii, 2015, Vol. 60, No. 12, pp. 1635–1640.
Rights and permissions
About this article
Cite this article
Dolin, S.P., Breslavskaya, N.N., Markov, A.A. et al. Mechanism and energetics of 1,2-addition of dioxygen 1O2(1Δg) to ethylene. Russ. J. Inorg. Chem. 60, 1495–1500 (2015). https://doi.org/10.1134/S0036023615120104
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S0036023615120104