Abstract
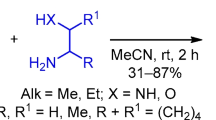
The reactions of aliphatic β-amino-β-trifluoromethylvinyl ketones with an excess of ethylenediamine at room temperature afforded the corresponding 2,3-dihydro-1H-1,4-diazepines or substituted 2-acetonyl-2-trifluoromethylimidazolidines (the latter were obtained when the approach to the carbonyl group was sterically hindered).
Similar content being viewed by others
References
K. I. Pashkevich, A. Ya. Aizikovich, and I. Ya. Postovskii,Izv. Akad. Nauk SSSR, Ser. Khim. 1981, 455 [Bull. Acad. Sci. USSR, Div. Chem. Sci., 1981,30 (Engl. Transl.)].
G. M. J. Slusarczuk and M. M. JoullieJ. Org. Chem., 1971,36, 37.
V. Ya. Sosnovskikh and M. Yu. Mel'nikov,Mendeleev Commun., 1998, 19.
H. W. Wanzlick and W. Lochel,Chem. Ber., 1953,86, 1463.
T. H. Fife and J. E. C. Hutchins,J. Am. Chem. Soc., 1976,98, 2536.
V. Ya. Sosnovskikh and M. Yu. Mel'nikov,Zh. Org. Khim., 1998,34, 303 [Russ. J. Org. Chem., 1998,34 (Engl. Transl.)].
Author information
Authors and Affiliations
Additional information
Translated fromIzvestiya Akademii Nauk. Seriya Khimicheskaya, No. 11, pp. 2305–2308, November, 1998.
Rights and permissions
About this article
Cite this article
Sosnovskikh, V.Y., Mel'nikov, M.Y. & Kovaleva, I.A. Interactions of aliphatic β-amino-β-trifluoromethylvinyl ketones with ethylenediamine. Russ Chem Bull 47, 2234–2237 (1998). https://doi.org/10.1007/BF02494288
Received:
Issue Date:
DOI: https://doi.org/10.1007/BF02494288