Summary
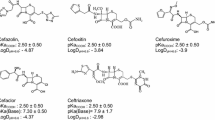
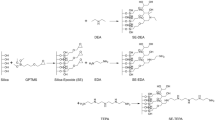
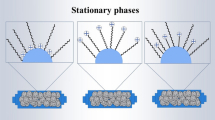
The partition coefficients, logP app RPLC, for a series of propranolol analogues have been determined by reversed phase high performance liquid chromatography. The hydrochloride salts and free base logP app RPLC show that charged and uncharged species can partition into the reversed phase. An extrathermodynamic relationship was found for logP app RPLC measured in two different column stationary phases, C8 and PMOS, suggesting that the same set of compounds experience a similar trend in these elution systems. The importance of using phosphate and/or MOPS buffer is due to the fact that the latter can avoid ion-pairing partitioning, but the former does play the same role for PMOS column when the free base is considered.
Similar content being viewed by others
References
Reymond, F.; Chopineaux-Courtois, V.; Steyaert, G.; Bouchard, G.; Carrupt, P.A.; Testa, B.; Girault, H.H.J. Electroanal. Chem. 1999,462, 235–250.
Avdeef, A.; Box, K.J.; Comer, J.E.A.; Hibbert, C.; Tam, K.Y.Pharm. Res. 1998,15, 209–215.
Fruttero, R.; Caron, G.; Fornatto E.; Boschi, D.; Ermondi, G.; Gasco, A.; Carrupt, P.A.; Testa, B.Pharm. Res. 1998,15, 1407–1413.
Ohshima, T.; Takagi, K.; Miyamoto, K.J. Liq. Chromatogr. 1993,16, 3933–3939.
El Tayar, N.; Testa, B.; Van de Waterbeemd, H.; Carrupt, P.A.; Kaumann, A.J.J. Pharm. Pharmacol. 1988,40, 609–612.
Recanatini, M.J. Pharm. Pharmacol. 1992,44, 68–70.
Pagliara, A.; Carrupt, P. A.; Caron, G.; Gaillard, P.; Testa, B.Chem. Rev. 1997,97, 3385–3400.
Barbato, F.; Caliendo, G.; Larotonda, M.I.; Morrica, P.; Silipo, C.; Vittoria, A.Farmaco 1990,45, 647–663.
Takacs-Novak, K.; Szasz, G.Pharm. Res. 1999,16, 1633–1638.
Britto, M.M.; Cass, Q.B.; Montanari, C.A.; Aboul-Enein, H.Y.J. Liq. Chrom. Rel. Technol.,1999,22, 2139–2149
Van de Waterbeemd, H.; Kansy, M.; Wagner, B.; Fischer, H. Lipophilicity in Drug Action and Toxicology inMethods and Principles in Medicinal, Chemistry: Mannhold, R.; Kubinyi, H.; Timmerman, H. Eds., Vol. 4, p. 73, VCH Weiheim,1996.
Anazawa, T.A.; Jardim, I.C.S.F.J. Liq. Chrom. Rel. Technol. 1998,21, 645–655, (b) Da Silva, M.C.H.; Jardim, I.C.S.F.J. Liq. Chrom. Rel. Technol. 1998,21, 2447–2458.
Avdeef, V.; Lipophilicity in Drug Action and Toxicology inMethods and Principles in Medicinal Chemistry: Mannhold, R.; Kubinyi, H.; Timmerman, H.; Pliška, V.; Testa, B.; van de Waterbeemd, H. Eds., Vol. 4, p. 109–139, VCH, Weinheim,1996.
Hansch, C.; Leo, A.; Hoekman, D. inExploring QSAR: Hydrophobic, Electronic, and Steric Constants, p. 143, ACS, Washington, DC1995.
PALLAS, CompuDrug.
Dorsey, J.G.; Cooper, W.T.; Siles, B.A.; Foley, J.P.; Barth, H.G.Anal. Chem. 1998,70, 591R-644R.
Selassie, C.D.; Hansch, C.; Khwajia, T.A.J. Med. Chem. 1990,33, 1914–1919.
Ferrari, S.; Leemann, T.; Dayer, P.Life Sci. 1991,48, 2259–2265.
Karlsson, J.; Artursson, P.Int. J. Pharm. 1991,71, 55–64.
Leo, A.; Hansch, C.; Elkins, D.Chem. Rev. 1971,71, 525.
Corradini; D.; Kalghatgi, K.; Horvath, C.J. Chromatogr. A 1996,728, 225–233.
Vailaya, A.; Horvath, C.J. Phys. Chem. B 1997,101, 5875–5888.
Hansch, C.; Leo, A. InExploring QSAR. Fundamentals and Applications in Chemistry and Biology, p. 158, ACS, Washington, DC,1995.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Britto, M.M., Cass, Q.B., Jardim, I.C.S.F. et al. The role of ion pairing in the chromatographic study of propranolol analogues. Chromatographia 53, 11–16 (2001). https://doi.org/10.1007/BF02492420
Received:
Revised:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF02492420