Abstract
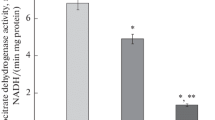
Activities of key carbohydrate-metabolizing enzymes in the livers of rats after bile duct ligation were studied during the recovery phase, after releasing the bile duct from ligation as an external biliary drainage. Enzyme activities were analyzed on the 3rd and 7th days after ligation and on the 3rd and 7th days after releasing the ligation. The altered enzyme activities, which resulted from the ligation of the bile duct, recovered after its release, although the recovery was delayed as when compared with those in the serum liver function tests. Glucokinase was the enzyme which was most delayed in recovery. The decreased activity of glucokinase had not returned to normal, even on the 7th day after release of the bile duct from its 7 day-ligation, whereas all the other enzyme activities had returned to normal by this time. It was also suggested that the decreased activity of glucokinase, which regulates the glucose uptake by the liver, was related to the glucose intolerance in obstructive jaundice. Accordingly, glucokinase can be used as a marker for determining the time of operation after biliary drainage.
Similar content being viewed by others
References
Ozawa K, Ida T, Yamada T, Yamaoka Y, Takasan H, Honjo I. Oral glucose tolerance in patients with jaundice. Surg Gynecol Obstet 1975; 140: 582–588.
Kobayashi M, Muto M, Shimada H, Shinmyo K, Abe T, Kito F, Kure H, Tsuchiya S. Relationship between glucose tolerance, insulin response and prognosis in patients with obstructive jaundice. Nippon Shokaki Geka Gakkai Zasshi (Jpn J Gastroenterol Surg) 1982; 15: 486–490. (English Abst.)
Soler NG, Exon PD, Paton A. Carbohydrate tolerance and insulin responses in obstructive jaundice. Brit Med J 1974; 4: 447–449.
Denk H, Eckerstorfer R, Rohr HP. The endoplasmic reticulum of the rat liver cell in experimental mechanical cholestasis. Correlated biochemical and ultrastructural-morphometric studies on structure and enzyme composition. Experimental and Molecular Pathology 1977; 26: 193–203.
Kurose M. Studies on liver cell injury in obstructive jaundice: Key enzyme activities of carbohydrate metabolism in rat after bile duct ligation. Nippon Geka Gakkai Zasshi (J Jpn Surg Soc) 1984; 85: 457–467. (English Abst.)
Ohyanagi H, Mitsuno T. Studies of the timing for the release of obstruction in patients with obstructive jaundice. In: Namiki M eds. Heisokusei-Odan o Meguru shomondai (Problems in obstructive jaundice). Tokyo: Igakutosho Publishers, Inc., 1976; 182–198. (in Japanese)
Ohyanagi H, Shirakawa M, Yamashita S, Sekita M, Okumura S, Nakaya S, Tei M, Nagatsu M, Mitsuno T. Biochemical approach to the obstructive jaundice to investigate the correct time for operation. Nippon Shokaki Geka Gakkai Zasshi (Jpn J Gastroenterol Surg) 1975; 8: 211–219. (English Abst.)
Shirakawa Y. Hepatic bile production and bile acid metabolism after relief of biliary obstruction. Nippon Geka Gakkai Zasshi (J Jpn Surg Soc) 1981; 82: 633–646. (English Abst.)
Ozawa K. Changes of bile contents and reduction of jaundice by percutaneous transhepatic cholangial drainage in obstructive jaundice. Nippon Geka Gakkai Zasshi (J Jpn Surg Soc) 1979; 80: 916–930. (English Abst.)
Denning DA, Ellison EC, Carely LC. Preoperative percutaneous transhepatic biliary decompression lowers operative morbidity in patients with obstructive jaundice. Am J Surg 1981; 141: 61–65.
Taketa K, Tanaka A, Watanabe A, Takesue A, Aoe H, Kosaka K. Undifferentiated patterns of key carbohydrate-metabolizing enzymes in injured liver. I. Acute carbon-tetrachloride intoxication of rat. Enzyme 1976; 21: 158–173.
Taketa K, Shimamura J, Takesue A, Tanaka K, Kosaka K. Undifferentiated patterns of key carbohydrate-metabolizing enzymes in injured liver. II. Human viral hepatitis and cirrhosis of the liver. Enzyme 1976; 21: 200–216.
Koide H, Oda T. Pathological occurrence of glucose 6-phosphatase in serum in liver disease. Clin Chim Acta 1959; 4: 554–561.
Lowrey OH, Resenbrough NJ, Farr AL, Randall RJ. Protein measurement with the folin phenol reagent. J Biol Chem 1951; 193: 265–275.
Viñuela E, Salas M, Sols A. Glucokinase and hexokinase in liver in relation to glycogen synthesis. J Biol Chem 1963; 238: PC 1175–1177.
Taketa K, Kaneshige Y, Tanaka A, Kosaka K. Differential responses of glucose 6-phosphate dehydrogenase and lipogenesis to fat-free diets. Biochem Med 1970; 4: 531–545.
Pogell BM, Tanaka A, Siddors RC. National activators for liver fructose 1,6-diphosphatase and the reversal of adenodine 5′-monophosphate inhibition by muscle phosphofructokinase. J Biol Chem 1968; 243: 1356–1367.
Taketa K, Pogell BM. Allosteric inhibition of rat liver fructose 1, 6-diphosphatase by adenosine 5′-monophosphate. J Biol Chem 1965; 240: 651–662.
Tanaka T, Harano Y, Sue F, Morimura H. Crystallization, characterization and metabolic regulation of two types of pyruvate kinase isolated from rat liver tissue. J Biochem 1967; 62: 71–90.
Sussor WS, Rutter WJ. Some distinctive properties of pyruvate kinase purified from rat liver. Biochem Biophys Res Commun 1968; 30: 14–20.
Malloy HT, Evelyn KA. Determination of bilirubin with photoelectric colorimeter. J Biol Chem 1937; 119: 481–490.
Reitmann S, Frankel S. A colorimetric method for the determination of serum glutamic oxalacetic and glutamic pyruvic transaminases. Am J Clin Pathol 1957; 28: 56–63.
Kind PRN, King EJ. Estimation of plasma phosphatase by determination of hydrolysed phenol with amino-antipyrine. J Clin Path 1954; 7: 322–326.
Wakui K, Kumada H, Muto N, Nagashima M, Kumagai T, Ito K. Progress in biochemical diagnosis. Igaku-no-Ayumi 1973; 86: 574–581. (in Japanese)
Watanabe A, Taketa K. The medicinal liver injury from the aspect of the dysregulation of protein synthesis. In: Yamamoto, eds. Yakubutsusei-Kanshogai no Rinsho (Clinical aspect of drug-induced liver injury). Tokyo: Kanahara Publishers, 1976; 27–39. (in Japanese)
Taketa K, Tanaka A. Correlation between the activities of liver glucokinase and hexokinase and the glucose intolerance in liver disease. Kanzo (Acta Hepatologica Japonica) 1974; 15: 115–117. (in Japanese)
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Hirata, K., Mimura, H. & Orita, K. Alterations in key carbohydrate-metabolizing enzyme activities in rat livers following bile duct ligation and its release. The Japanese Journal of Surgery 18, 68–76 (1988). https://doi.org/10.1007/BF02470849
Received:
Issue Date:
DOI: https://doi.org/10.1007/BF02470849