Abstract
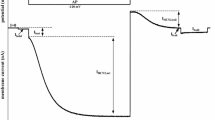
Barium currents through ion channels formed by α1-subunit of L-type Ca2+ channel (I α1) were recorded from cultured chinese hamster ovary (CHO) cells. The cells were stably transfected with either a cardiac or a smooth muscle (SM) variant of α1-subunit. TheI α1 in both cases exhibited similar fast voltage-dependent activation kinetics and slow apparent inactivation kinetics. With 10 mM Ba2+ in the bath solution,I α1 was activated at potentials more positive than −40 mV, peaked between 0 and +10 mV, and reversed at about +50 mV. In addition to slow apparent inactivation of inward current, both subunits provided an extremely slow voltage-dependent inactivation at potentials more positive than −100 mV, with half-maximum inactivation at −43.4 mV for cardiac and −41.4 mV for SM α1-subunits. The onset of inactivation as well as recovery from this process were within a time range of minutes. The voltage dependence of steady-state inactivation could be fitted by the sum of two Boltzmann's equations with slope factors of about 12 mV and 5 mV. A less sloped component has its midpoints at −75.6 and −63.7 mV, and a steeper component has its midpoints at −42.8 and −37.7 mV for cardiac and SM α1-subunits, respectively. Relative contribution of the steeper component was higher in both subunits (0.86 and 0.66 for cardiac and SM subunits, respectively). For comparison, the inactivation curves for 5-sec-long conditioning prepulses could be fitted by single Boltzmann's distribution with a 20 mV more positive midpoint and a slope factor of about 13 mV. In contrast to the steady-state inactivation curves, they showed considerable overlap with the steady-state activation curve. Our results reflect functional consequences of known sequence differences between α1-subunits of the cardiac and SM L-type Ca2+ channels and could be used in structural modeling of Ca2+ channel gating. In addition, they show that depolarization-induced window current has a transient nature and decays with the development of extremely slow inactivation. This is the first demonstration that slow inactivation of the L-type Ca2+ channel is an intrinsic property of its α1-subunits.
Similar content being viewed by others
References
R. Eckert and J. E. Chad, “Inactivation of Ca channels,”Prog. Biophys. Mol. Biol.,44, 215–267 (1984).
E. Carbone and D. Swandulla, “Neuronal Ca channels: kinetics, blockage and modulation,”Prog. Biophys. Mol. Biol.,54, 31–58 (1989).
K. Jmari, S. Mironneau, and T. Mironneau, “Inactivation of calcium channel current in rat uterine smooth muscle: evidence for calcium and voltage-dependant mechanisms,”J. Physiol.,380, 111–126 (1986).
K. S. Lee, E. Marban, and R. W. Tsien, “Inactivation of calcium channels in mammalian heart cells: joint dependence on membrane potential and intracellular calcium,”J. Physiol.,364, 395–411 (1985).
B. Nilius, T. Kitamura, and H. Kuriyama, “Properties of inactivation of calcium channel currents in smooth muscle cells of rabbit portal vein,”Pflügers Arch.,426, 239–246 (1994).
G. Varadi, Y. Mori, G. Mikala, and A. Schwartz, “Molecular determinants of Ca2+ channel function and drug action,”TiPS,16, 43–49 (1995).
M. Biel, R. Hullin, St. Freunder, et al., “Tissue specific expression of high voltage activated dihydropyridine-sensitive L-type calcium channels,”Eur. J. Biochem.,200, 81–88 (1991).
A. Welling, Y. W. Kwan, E. Bosse, et al., “Subunit-dependent modulation of recombinant L-type calcium channels,”Circ. Res.,73, 974–980 (1993).
O. P. Hamil, A. Marty, E. Neher, et al., “Improved patch-clamp techniques for high resolution current recording from cells and cell-free membrane patches,”Pflügers Arch.,391, 85–100 (1981).
V. A. Buryi, N. Morel, S. Salomone, et al., “Evidence for a direct interaction of thapsigargin with voltage-dependent Ca2+ channel,”Naunyn-Schmiedebrg's Arch. Pharmacol.,351, 40–45 (1995).
G. Isenberg and U. Klockner, “Calcium currents of isolated bovine ventricular myocytes are fast and of large amplitude,”Pflügers Arch.,395, 30–41 (1982).
R. L. Ruff, L. Simoncini, and W. Stuhgmer, “Slow sodium channel inactivation in mammalian muscle: a possible role in regulation excitability,”Muscle Nerve,11, 502–510 (1988).
A. Mikami, K. Imoto, T. Tanabe, et al., “Primary structure and functional expression of the cardiac dihydropyridine-sensitive calcium channel,”Nature,350, 398–402 (1989).
M. Biel, P. Ruth, E. Bosse, et al., “Primary structure and functional expression of high voltage activated calcium channel from rabbit lung,”FEBS Lett.,269, 409–412 (1990).
E. Perez-Reyes, X. Wei, A. Castellano, and L. Birnbaumer, “Molecular diversity of L-type calcium channels. Evidence for alternative splicing of the transcripts of three non-allelic genes,”J. Biol. Chem.,265, 20430–20436 (1990).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Bouryi, V.A. Extremely slow inactivation of the ion channels formed by transfected α2 of L-type Ca2+ channelsof L-type Ca2+ channels. Neurophysiology 30, 301–304 (1998). https://doi.org/10.1007/BF02462843
Issue Date:
DOI: https://doi.org/10.1007/BF02462843