Abstract
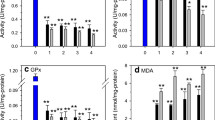
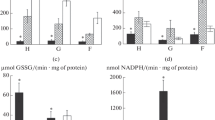
The protective effects of glutathione-dependent enzymes have been studied against cadmium toxicity in the liver and kidney of two fresh water fishesChanna punctatus andClarias batrachus. Specie's differences in the activity of tissue enzymes have also been studied. Cadmium treatment induced lipid peroxidation in the liver and kidney of both species, the kidney being the more susceptible. Enzymological observations revealed thatChanna punctatus is better equipped with conjugating enzymes thanClarias batrachus. Fish having higher activities of these enzymes are thus expected to withstand oxidative stress more effectively.
Similar content being viewed by others
Literature Cited
Aksnes, A. and L. R. Njaa. 1981. Catalase, glutathione peroridase and superoxide dismutase in different fish species. Comp. Biochem. Physiol., 69 B: 893–896.
Bend, J. R. and M. D. James. 1978. Xenobiotic metabolism in marine and fresh water species. Biochem. Biophys. Pessp. Mar. Biol., 4: 125.
Beutler, E. 1971. Abnormalities of the hexose monophosphate shunt. Semin. Hematol., 8: 311.
Braddon, S. A., C. M. Mc Ilvaine and J. E. Balthrop. 1985. Distribution of GSH and GSH cycle enzymes in black sea bass (Centropristis striata). Comp. Biochem. Physiol., 80 B: 213–216.
Carlbarg, L. and B. Mannervik. 1985. Methods in Enzymology, 113. Academic Press, New York. 484 pp.
Dierickx, P. J. 1981.In vitro inhibition of the soluble glutathione-S-transferase from rat liver by heavy metals. Enzyme., 27: 25–32.
Ellman, G. L. 1959. Tissue sulphydryl groups. Arch. Biochem. Biophys., 82: 70–77.
Finlayson, B. J. and K. M. Verrue. 1982. Toxicities of copper, zinc and cadmium mixtures to juvenile Chinook Salmon. Transactions of the American Fisheries Society., 111: 645–650.
Fisher, R. A. 1950. Statistical methods for research workers. 11th ed. Cliver and Boyd, London.
Flohe, L. and W. A. Gunzler. 1976. Glutathione dependent enzymatic oxido reduction reaction. Pages 17–34in I. M. Arias and W. B. Jakoby, eds. Glutathione metabolism and function. Raven Press, New York.
Flohe, L. 1982. Role of GSH peroxidase in lipid peroxide metabolism. Pages 149–159in K. Yogi, ed. Lipid peroxidation in Biology and Medicine. Academic Press, New York.
Graf, P. and H. Sies. 1984. Hepatic uptake of cadmium and its biliary release as affected by dithioerythritol and glutathione. Biochem. Pharmacol., 33: 639.
Habig, W. H., M. J. Pabst and W. B. Jakoby. 1974. Glutathione-S transferases. The first enzymatic step in mercapturic acid formation. J. Biol. Chem., 249: 7130–7139.
Jakoby, W. B., W. H. Habig, J. H. Keen, J. N. Kettey and M. J. Pabst. 1976. Glutathione-S-transferases: Catalytic aspects. Pages 189–202in I. M. Arias and W. B. Jacoby, eds. Glutathione metabolism and function. Raven Press, New York.
Jakoby, W. B. and W. H. Habig. 1980. Pages 63–94in W. B. Jakoby, ed. Enzymatic basis of detoxification. Academic Press, New York.
Jordan, R. A. and J. B. Shenkman. 1982. Relationship between malondialdehyde production and arachidonate consumption during NADPH-supported microsomal lipid peroxidation. Biochem. Pharmacol., 31: 1393–1400.
Kagi, J. H. R. and Y. Kojima. 1987. Metallothionein II. Experientia Suppl., Birhauser, Basel. pp. 627–630.
Kaplowitz, M. D. 1981. The importance and regulation of hepatic glutathione. The Yale J. Biol. Med., 54: 497–502.
Kettrer, B., B. Coles and D. J. Meyer. 1983. The role of glutathione in detoxication. Environ. Hlth. Perspect., 49: 56–69.
Lowry, O. H., N. J. Rosebrough, A. L. Farr and R. J. Randall. 1951. Protein measurement with the folin phenol reagent. J. Biol. Chem., 193: 265.
Meister, A. 1983. Selective modification of glutathione metabolism. Science, 220: 472.
Muramatsu, T., S. I. Iwanaga and S. Kan. 1980. Isolation and characterization of glutathione reductase from the dark muscle of pacific mackerel. Bull. Japan. Soc. Sci. Fish., 46: 757–762.
Rana, S. V. S. and S. Kumar. 1994. Antioxidative enzyme in the liver of rats after exposure to xylene, toluene and methyl alcohol separately and in combination. Physiol. Chem. Phys. and Med. NMR., 27: 25–29.
Rana, S. V. S. and S. Verma. 1996. Protective effects of GSH, vitamin E and selenium on lipid peroxidation in cadmium-fed rats. Biol. Trace. Element. Res., 51: 161–168.
Shukla, G. S., R. S. Srivastava and S. V. Chandra. 1987. Glutathione metabolism in liver, kidney and testis of rats exposed to cadmium. Ind. Health., 25: 134–146.
Shukla, G. S., T. Hussain and S. V. Chandra. 1987. Possible role of regional superoxide dismutase activity and lipid peroxide levels in cadmium neurotoxicity.In vivo andin vitro studies in growing rats. Life. Sci., 41: 2215–2221.
Shukla, G. S., T. Hussain, R. S. Srivastava and S. V. Chandra. 1989. Glutathione peroxidase and catalase in liver, kidney, testis and brain regions of rats following cadmium exposure and subsequent withdrawal. Industrial Health., 27: 59–69.
Tappel, M. E., J. Chaudiere and A. L. Tappel. 1982. Glutathione per-oxidase activities of animal tissues. Comp. Biochem. Physiol., 73B: 945–949.
Thomas, P. and H. W. Woffored. 1984. Effect of metals and organic compounds on hepatic glutathione, cysteine and acid soluble thiols levels in mullet. (Mugil cefolus L.) Toxic. Appl. Pharma., 50: 329–335.
Tsan, M. F., E. H. Danis, P. J. D. Vecchio and C. L. Rosano. 1985. Enhancement of intracellular glutathione protects endothelial cells against oxidative damage. Biochem. Biophys. Res. Communi., 127: 270.
Wendel, A. 1980. Page 333in W. B. Jakoby, ed. Enzymatic basis of detoxication. Academic Press, New York.
Author information
Authors and Affiliations
About this article
Cite this article
Rana, S.V.S., Singh, R. Specie's differences in glutathione-dependent enzymes in the liver and kidney of two fresh water fishes and their implications for cadmium toxicity. Ichthyological Research 43, 223–229 (1996). https://doi.org/10.1007/BF02347594
Received:
Revised:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF02347594