Summary
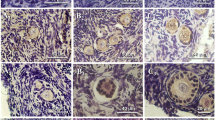
Immunohistochemical methods were used to show the presence and distribution of transforming growth factor-β1 and β2 during folliculogenesis in quail ovarian tissues. The results indicated that both transforming growth factor-β subtypes are present. Immunolabelling for transforming growth factor-β1 demonstrated that prelampbrush oocytes are immunoreactive in the Balbiani complex, and developing and pre-ovulatory oocytes in the zona radiata. Immunolabelling was also associated with granulosa cells. The number of stained granulosa cells decreased during folliculogenesis. In the pre-ovulatory follicles, immunolabelling was found predominantly in the theca interna. Immunolabelling for transforming growth factor-β2 was associated with the zona radiata of developing and preovulatory follicles, and with stromal interstitial cells. Moderate immunoreactivity was found in the Balbiani complex of prelampbrush oocytes. Weak immunolabelling was localized in the granulosa cells of prelampbrush follicles, and in a few cells of the theca interna of pre-ovulatory follicles. The immunolocalization of transforming growth factor-β1 and-β2 in the quail ovary supports their autocrine and/or paracrine role in avian ovarian processes.
Similar content being viewed by others
References
Arrick, B. A., Lee, A. L., Grendell, R. L. &Derynck, R. (1991) Inhibition of translation of transforming growth factor-β3 mRNA by its 5′ untranslated region.Mol. Cell. Biol. 11, 4306–13.
Callebaut, M. (1983) The constituent oocytal layers of the avian germ and the origin of the primordial germ cell yolk.Arch. Anat. Microsc. 72, 199–214.
Ding, J. &Foxcroft, G. R. (1994) Epidermal growth factor enhances oocyte maturation in pigs.Mol. Reprod. Dev. 39, 30–40.
Ellingsworth, L. R., Brennan, J. E., Fok, K., Rosen, D. M., Bentz, H., Piez, K. A. &Seyedin, S. M. (1986) Antibodies to the N-terminal portion of cartilageinducing factor A and transforming growth factor β. Immunohistochemical localization and association with differentiating cells.J. Biol. Chem. 261, 12362–7.
Graham, R. C. &Karnovsky, M. J. (1966) The early stages of absorption of injected horseradish peroxidase in the proximal tubules of mouse kidney: ultrastructural cytochemistry by a new technique.J. Histochem. Cytochem. 14, 291–302.
Gupta, S. K. &Gilbert, A. B. (1988) Mast cells in the ovary ofGallus gallus domesticus.Br. Poultry Sci. 29, 245–9.
Hausen, P. &Dryer, C. (1982) Urea reactivates antigens in paraffin sections for immunofluorescent staining.Stain Technol. 57, 321–4.
Heine, U. I., Munoz, E. F., Flanders, K. C., Ellingsworth, L. R., Lam, H.-Y.P., Thompson, N. L., Roberts, A. B. &Sporn, M. B. (1987) Role of transforming growth factor-β in the development of the mouse embryo.J. Cell. Biol. 105, 2861–76.
Holzwarth, M. A. &Sawetawan, C. (1985) Postnatal development of serotonin in adrenal medullary cells.Brain Res. Bull. 14, 15–23.
Jakowlew, S. B., Dillard, P. J., Sporn, M. B. &Roberts, A. B. (1990) Complementary deoxyribonucleic acid cloning of an mRNA encoding transforming growth factor-β2 from chicken embryo chondrocytes.Growth Factors 2, 123–33.
Kingsley, D. M. (1994) The TGF-β superfamily: new members, new receptors, and new genetic tests of function in different organisms.Genes Devel. 8, 133–46.
Koike, S. &Noumura, T. (1993) Immunohistochemical localizations of TGF-β in the developing rat gonads.Zool. Sci. 10, 671–7.
Ksander, G. A., Gerhardt, C. O., Dasch, J. R. &Ellingsworth, L. R. (1990) A novel polyclonal antibody (CLB1/29) for immunolocalization of transforming growth factor-β2 (TGF-β2) in adult mouse.J. Histochem. Cytochem. 38, 1831–40.
Lafrance, M., Croze, F. &Tsang, B. K. (1993a) Influence of growth factors on the plasminogen activator activity of avian granulosa cells from follicles at different maturational stages of preovulatory development.J. Mol. Endocrinol. 11, 291–304.
Lafrance, M., Zhou, L. &Tsang, B. K. (1993b) Interactions of transforming growth factor-α and-β and luteinizing hormone in the regulation of plasminogen activator activity in avian granulosa cells during development.Endocrinology 133, 720–7.
Law, A. S., Burt, A. D. &Armstrong, D. G. (1995) Expression of transforming growth factor-β mRNA in chicken ovarian follicular tissue.Gen. Comp. Endocrinol. 98, 227–33.
Li, J., Li, M., Lafrance, M. &Tsang, B. K. (1994) Avian granulosa cell prostaglandin secretion is regulated by transforming growth factor α and β and does not control plasminogen activator activity during follicular development.Biol. Reprod. 51, 787–94.
Li, M., Morley, P., Asem, E. K. &Tsang, B. K. (1991) Epidermal growth factor elevates intracellular pH in chicken granulosa cells.Endocrinology 129, 656–62.
Martinez, A., Miller, M.-J., Quinn, K., Unsworth, E. J., Ebina, M. &Cuttitta, F. (1995) Non-radioactive localization of nucleic acids by directin situ PCR andin situ RT-PCR in paraffin-embedded sections.J. Histochem. Cytochem. 43, 739–47.
Massagué, J. (1990) The transforming growth factor-β family.Ann. Rev. Cell Biol. 6, 597–641.
Matsuyama, S. &Takahashi, M. (1995) Immunoreactive (ir)-transforming growth factor (TGF)-β in rat corpus luteum: ir-TGFβ is expressed by luteal macrophages.Endocrinol. J. 42, 203–17.
Mori, T. (1990) Immuno-endocrinology of cyclic ovarian function.Am. J. Reprod. Immunol. 23, 80–9.
Mulheron, G. W. &Schomberg, D. W. (1993) The intraovarian transforming growth factor system. InThe Ovary. (edited byAdashi, E. Y. &Leung, P. C. K.) pp. 337–61. New York: Raven Press Ltd.
Parshad, R. K. &Kathpalia, K. (1993) Distribution and characteristics of mast cells in the chick ovary.Br. Poultry Sci. 34, 65–71.
Roy, S. K. (1993) Epidermal growth factor and transforming growth factor-β modulation of follicle-stimulating hormone-induced deoxyribonucleic acid synthesis in hamster preantral and early antral follicles.Biol. Reprod. 48, 552–7.
Shull, M. M. &Doetschman, T. (1994) Transforming growth factor-β1 in reproduction and development.Mol. Reprod. Dev. 39, 239–46.
Tilly, J. L. &Johnson, A. L. (1990) Modulation of hen granulosa cell steroidogenesis and plasminogen activator activity by transforming growth factor alpha.Growth Factors 3, 247–55.
Van Nassauw, L., Callebaut, M., Harrisson, F., Daneels, G. &Moeremans, M. (1989) Immunohistochemical localization of desmin in the quail ovary. Demonstration of a suspensory apparatus.Histochemistry 90, 371–7.
Van Nassauw, L., De Deurwaerder, R., Leeuwesteyn, A., Harrisson, F. &Callebaut, M. (1995) Immunohistochemical localization of epidermal growth factor in the ovary of the adult Japanese quail.Histochem. J. 27, 890–6.
Wight, P. A. L. (1970) The mast cells ofGallus domesticus. 1. Distribution and ultrastructure.Acta Anat. 25, 100–13.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Van Nassauw, L., Schrevens, A., Harrisson, F. et al. Immunohistochemical localization of transforming growth factor-β1 and β2 during folliculogenesis in the quail ovary. Histochem J 28, 859–865 (1996). https://doi.org/10.1007/BF02331389
Received:
Revised:
Issue Date:
DOI: https://doi.org/10.1007/BF02331389