Abstract
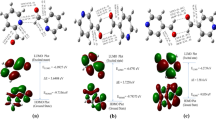
UV spectra of 2,4-dihalopicolines and their N-oxides are presented, and the influence of substituents on spectral parameters is discussed. The electronic spectra were calculated by a modified INDO method. Transition energies, intensities, and assignments were compared with UV spectra. The degree of intramolecular charge transfer in 2,4-dihalopicolines is greater than in 2-halopicoline N-oxides and smaller than in 2-halo-4-nitropicoline N-oxides. Difference values of HOMO-LUMO energies point out that the susceptibility on photochemical reaction lies in the order: 2-halo-4-nitropicoline N-oxides > 2,4-dihalopicoline N-oxides > 2-halopicoline N-oxides > pyridine N-oxide.
Similar content being viewed by others
References
E. Evleth, Theor. Chim. Acta,11, 145 (1968).
Ha Tae-Kyu, Theor. Chim. Acta,43/4 337 (1977).
G. V. Kulkarni and A. Ray, J. Mol. Struct.,71, 253 (1981).
C. Leibovici and J. Streith, Tetrahed. Lett.N5, 387 (1971).
K. Seibold, G. Wagniere, and H. Labhard, Helv. Chim. Acta.,52, 789 (1969).
M. Cigniti and L. Soccorsi, Spectrochim. Acta.,41A,11, 1287 (1965).
L. Chmurzyiński and A. Liwo, J Mol. Struct.,218, 129 (1990).
A. Puszko, Chem. Pap.,44/3, 313 (1990).
A. Puszko, Chem. Pap.,45/5, 621 (1990).
A. Puszko, Magn. Res. Chem.,30, 271 (1992).
A. Puszko, Polish J. Chem.,66, 1615 (1992).
A. Puszko, Polish J. Chem.,66, 1979 (1992).
A. Puszko, J. Crystall. Spectrosc. Res.,23/1, 23 (1993).
A. Puszko, Polish. J. Chem.,67, 2005 (1993).
A. Puszko, Polish J. Chem.,67, 837 (1993).
A. Puszko, Polish J Chem.,68, 657 (1994).
A. Puszko, J. Mol. Struct.,344, 1 (1995).
A. Puszko and Z. Talik, Prace Naukowe AE.,167/189, 179 (1980).
A. Puszko and Z. Talik, Polish J. Chem.,65, 377 (1990).
K. Fukui, A. Imamura, and C. Negata, Bull. Chem. Soc. Jpn.,33, 122 (1960).
I. I. Okabayoshi, J. Ferment. Technol.,31, 373 (1953).
G. Berthier, M. Defranceschi, and P. Lazzeretti, J. Mol. Struct.,254, 205 (1992).
J. Zyss, D. S. Chemla, and J. F. Nicoud, J. Chem. Phys.,74, 4800 (1981).
J. A. Pople and D. L. Beveridge, Approximate Molecular Orbital Theory, McGraw-Hill, New York (1970).
J. Lipiński and J. Leszczyiński, Int. J. Quantum Chem.,22, 253 (1983).
J. Lipiński, A. Nowak, and H. Chojnacki, Acta Phys. Polon., A.,53, 229 (1978).
J. Lipiński, J. Quantum Chem.,34, 423 (1988).
M. J. S. Dewar, E. G. Zoebish, and E. F. Healy, J. Am. Chem. Soc.,107, 3902 (1985).
Additional information
Department of Organic Chemistry, University of Economics, PL-53-342 Wroclaw, Poland. Translated from Khimiya Geterotsiklicheskikh Soedinenii, Vol. 34, No. 10, pp. 1352–1366, October, 1999.
Rights and permissions
About this article
Cite this article
Puszko, A. Electronic spectra and structure of 2,4-dihalopicolines and their N-oxides. Chem Heterocycl Compd 34, 1148–1160 (1998). https://doi.org/10.1007/BF02319493
Received:
Issue Date:
DOI: https://doi.org/10.1007/BF02319493