Abstract
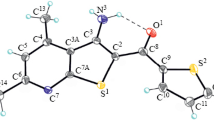
Condensation of aliphatic aldehydes with cyanothioacetamide has given 4-alkyl-6-amino-3,5-dicyano-2(1H)-pyridinethiones, which have also been synthesized by recyclization of 4-alkyl-2,6-diamino-4H-thiopyrans. Substituted 2-alkylthiopyridines and thieno[2,3-b]pyridines have been prepared from the pyridinethiones. 2,6-Diamino-4-isopropyl-3,5-dicyano-4H-thiopyran and 6-amino-4-isobutyl-2-methylthio-3,5-dicyanopyridine have been studied by x-ray crystallography.
Similar content being viewed by others
References
N. G. Frolova, V. K. Zav'yalova, and V. P. Litvinov, Izv. Russ. Akad. Nauk., Ser. Khim., No. 4, 938 (1996).
V. G. Kul'nevich, E. A. Kaigorodova, I. S. Arustamova, L. V. Korobchenko, G. V. Vladyko and E. I. Boreko, Khim.-farm. Zh., No. 2, 132 (1990).
V. Hagen, E. Klauschenz, A. Rumler, A. Hagen, S. Heer, R. Nitzner, H. Niedrich, and D. Lohmann, Pharmazie,45, 189 (1990).
V. Hanfeld, S. Leistner, G. Wagner, D. Lohmann, H. Hoppe, and S. Heer, Pharmazie,44, 12 (1989).
A. Rumler, V. Hagen, and A. Hagen, Pharmazie,45, 657 (1990).
W. S. Saari, J. S. Wai, T. E. Fisher, C. M. Thomas, J. M. Hoffman, C. S. Rooney, A. M. Smith, J. H. Jones, D. L. Bamberger, M. E. Goldman, J. A. O'Brien, J. H. Nunberg, J. C., Quintero, W. A. Schleif, E. A. Emini, and P. S. Anderson, J. Med. Chem.,35, 3792 (1992).
J. S. Wai, T. M. Williams, D. L. Bamberger, T. E. Fisher, J. M. Hoffman, R. J. Hudcosky, S. C. MacTough, C. S. Rooney, W. S. Saari, C. M. Thomas, M. E. Goldman, J. A. O'Brien, E. A. Emini, J. H. Hunberg, J. C. Quintero, W. A. Schlief, and P. S. Anderson, J. Med. Chem.,36, 249 (1993).
Yu. A. Sharanin, A. M. Shestopalov, V. N. Nesterov, S. N. Melenchuk, V. K. Promonenkov, V. E. Shklover, Yu. T. Struchkov, and V. P. Litvinov, Zh. Org. Khim.,25, 1323 (1989).
V. D. Dyachenko, V. N. Nesterov, Yu. T. Struchkov, Yu. A. Sharanin, and V. E. Shklover, Zh. Obshch. Khim.,59, 881 (1989).
J. Bellanato, F. Florencio, S. G. Blanco, N. Martin, and C. Seoane, J. Mol. Struct.,19, 162 (1987).
F. H. Allen, O. Kennard, D. G. Watson, L. Brammer, A. G. Orpen, and R. Taylor, J. Chem. Soc., Perkin Trans. II, No. 12, S1 (1987).
A. Bondi, J. Phys. Chem.,70, 3006 (1966).
W. Robinson and G. M. Sheldrick, Crystallographic Computing. Techniques and New Technologies, Oxford University Press (1988), p. 366.
Additional information
T. G. Shevchenko State Teaching Institute, Lugansk 348011. A. N. Nesmeyanov Institute of Organoelemental Compounds, Moscow 117813. N. D. Zelinskii Institute of Organic Chemistry, Russian Academy of Sciences, Moscow 117913. Translated from Khimiya Geterotsiklicheskikh Soedinenii, No. 12, pp. 1655–1663, December, 1997.
Rights and permissions
About this article
Cite this article
Dyachenko, V.D., Krivokolysko, S.G., Nesterov, V.N. et al. Synthesis of 4-alkyl-6-amino-3,5-dicyano-2(1H)-pyridinethiones. Chem Heterocycl Compd 33, 1430–1437 (1997). https://doi.org/10.1007/BF02291644
Received:
Issue Date:
DOI: https://doi.org/10.1007/BF02291644