Abstract
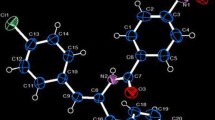
The synthesis of the energetic compound 4-[(4-nitro-1,2,5-oxadiazol-3-yl)-NNO-azoxyl]-1,2,5-oxadiazol-3-amine (3) was achieved in two steps from diaminofurazan (1). Compound 3 was characterized by X-ray diffraction. From the X-ray structure a bifurcated intramolecular H bond between O(2)-H(2)-N(4) was observed. In addition, intramolecular H bonding was observed between H(1) and N(7)′ of an adjacent molecule. One molecule of ethanol and one-half molecule of water per molecule of3 was observed in the crystal lattice. However, no H bonding was observed between the solvent molecules and3 in the crystal lattice. Despite the presence of solvent in the crystal lattice,3 was found to have a high crystal density (d=1.856 g/cm3).
Similar content being viewed by others
References
Stoner, C. E., Jr.; Brill, T. B.Combustion Flame 1991,83, 302.
Brill, T. B.Prog. Energy Combust. Sci. 1992,18, 91–116.
Williams, G. K.; Palopoli, S. F.; Brill, T. B.Combustion Flame 1994,98, 197–204.
Gunasekaran, A.; Trudell, M. L.; Boyer, J. H.Heteroatom Chem. 1994,5/6, 441.
Data obtained from Stephen A. Aubert, Eglin Air Force Base, Florida.
Meyer, R.Explosives; VCH: Weinheim, 1987, p. 266.
Novikova, T. S.; Melnikova, T. M.; Kharitonova, O. V.; Kulagina, V. O.; Aleksandrova, N. S.; Sheremetev, A. V.; Pivina, T. S.: Khmelnitskii, L. I.; Novikov, S. S.Mendeleev Commun. 1994, 138.
Cady, H. H.; Carson, A. C.Acta Crystallogr. 1967,23, 601.
Urbanski, T.; Vasudeva, S. K.J. Sci. Ind. Res. 1978,37, 250.
The densityd(g/cm3), detonation velocityD (mm/μsec), and detonation pressurePCJ (kbar) were computed with a program (Dickerson Method) obtained from the Naval Weapons Center, China Lake, CA.
Gunasekaran, A.; Jayachandran, T.; Boyer, J. H.; Trudell, M. L.J. Heterocycl. Chem. 1995,32, 1405.
Solodyuk, G. D.; Boldyrev, M. D.; Gidaspov, B. V.; Nikolaev, V. D.Zh. Org. Khim. 1981,17, 861 [English translation, p. 756].
The authors have deposited the crystallographic data for3 at the Cambridge Crystallographic Data Centre, University Chemical Laboratory, Lensfield Road, Cambridge, CB2 1EW, U.K.
Frenz, B. A. InComputing in Crystallography; Schenk, M.; Oltholf-Hazekamp, R.; van Koningsveld, M.; Bassi, G. C., eds.; Delft University Press: Delft, The Netherlands, 1982.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Zelenin, A.K., Stevens, E.D. & Trudell, M.L. Synthesis and structure of 4-[(4-Nitro-1,2,5-oxadiazol-3-yl)-NNO-azoxyl]-1, 2,5-oxadiazol-3-amine. Struct Chem 8, 373–377 (1997). https://doi.org/10.1007/BF02281251
Received:
Revised:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF02281251