Abstract
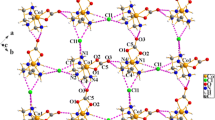
[Cr(en)2CO3]I (I), ICoO3N4C5H16, crystallizes from water at 21°C in space groupP21/c (no. 14), with lattice constantsa=7.298(4),b=8.622(8),c=17.577(6)Å,β=91.29(4)°;V=1105.59 Å3 andd(calc; MW=359.11, Z=4)=2.157 g cm−3. A total of 2825 data points were collected over the range of 4°≤2θ≤50°; of these, 1855 (independent and withI≥3σ(I)) were used in the structural analysis. Data were corrected for absorption (μ=37.657 cm−1) and the transmission coefficients ranged from 0.4850 to 0.9991. The finalR(F) andR w(F) residuals were, respectively 0.134 and 0.113. The cations exist in the lattice as the enantiomeric pair Λ(δδ) and Δ(λλ). NH4{[cis-β-Co(trien)CO3]2}(PF6)3 (II), Co2P3F18O6N9C14H40, crystallizes from water at 21 °C in space groupP21/c (no. 14), with lattice constantsa=10.397(2),b=20.292(3),c= 27.082(4) Å,β=100.30(3)°;V=3545.70 Å3 andd(calc; MW=983.29, Z=4)=1.842 g cm−3. A total of 3724 data were collected over the range of 4°≤2θ≤50°; of these, 2653 (independent and withI≥3σ(I)) were used in the structural analysis. Data were corrected for absorption (μ=12.031 cm−1) and the transmission coefficients ranged from 0.8326 to 0.99985. The finalR(F) andR w (F) residuals were, respectively 0.104 and 0.124. The cations exist in the asymmetric unit asΛ(δδδ) andΔ(λδλ)[cis-β-Co(trien)CO3]+ pairs. The three independent PF6 − anions exhibit the usual high thermal motion typical of these species and the NH4 + cation is either disordered or exhibits high thermal motion also (its H atoms could not be found in difference maps).
Similar content being viewed by others
References
Gargallo, M. F.; Mather, J. D.; Duesler, E. N.; Tapscott, R. E.Inorg. Chem. 1983,22, 2888.
Loehlin, J. H.; Fleisher, E. B.Acta Crystallogr. Sect. B 1976,32, 3063.
K. Yamanari, private communication to I. Bernai, December 3, 1987.
Bigoli, F.; Lafranchi, M.; Leporati, E.; Pellighelli, M. A.Cryst. Struct. Commun. 1980,9, 1261.
Bernal, I.; Myrczek, J.; Cetrullo, J.; Massoud, S. S.J. Coord. Chem. 1993,30, 29.
MacDonald, D. J.; Garner, C. S.J. Am. Chem. Soc. 1961,83, 4125.
Sargeson, A. M.; Searle, G. H.Inorg. Chem. 1967,6, 787.
Roof, R. B.A Theoretical Extension of the Reduced Cell Concept in Crystallography, Report LA-4038; Los Alamos Scientific Laboratory, 1969.
Cromer, D. T.; Waber, J. T.International Tables for X-ray Crystallography; The Kynoch Press; Birmingham, England, 1975; vol. IV, Tables 2.2.8 and 2.3.1, respectively, for the scattering factor curves and the anomalous dispersion values.
Bernal, I.; Cetrullo, J.Inorg. Chim. Acta 1986,120, 109.
Bernal, I.; Cai, J.; Myrczek, J.J. Am. Chem. Soc. (submitted).
Yamanari, K.; Fuyuhiro, A.J. Chem. Soc., Dalton 1991, 2903.
Bernal, I.; Myrczek, J.; Cai, J.Polyhedron. 1993,12, 1149.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Bernai, I., Cai, J., Cetrullo, J. et al. The phenomenon of conglomerate crystallization: XXXVII: The crystallization behavior of the Cr(III) and Co(III) amine carbonates [Cr(en)2CO3]I (I) and NH4{[cis-β-Co(trien)Co3]2}(PF6)3 (II). Struct Chem 5, 265–275 (1994). https://doi.org/10.1007/BF02275499
Received:
Revised:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF02275499