Summary
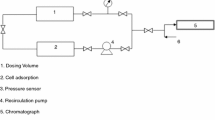
Rate constants for bimolecular reactions in the gas phase, under diffusion controlled conditions, can easily be determined by the reversed-flow gas chromatography (RF-GC) technique. The analysis of the diffusion band by means of a simple PC programme gives directly an apparent, second-order rate constant for gaseous reactions. By varying the amounts of the reactants, one can calculate the true order of the reaction and the true non-first-order rate constant of gaseous reactions. The calibration problem of the analytical techniques in non-first-order reaction kinetics is absent as are other disadvantages connected with carrier gas flow, peak shape and their instrumental spreading. The method can be used for atmospheric reactions and was applied in the gaseous reaction systems: SO2+NO2, SO2+Br2, C6H6+NO2, C6H5CH3+NO2 and C3H6+NO2 with various concentrations of reactants in nitrogen. The effect of the NO2 concentration on the apparent second-order rate constant of C2H4+NO2 at 333.2 K was also studied. Finally, the effect of sun light pre-irradiation of C2H2+NO2 in nitrogen was investigated.
Similar content being viewed by others
References
N. A. Katsanos, I. Georgiadou, J. Chem. Soc. Chem. Commun. 242 (1980).
N. A. Katsanos, “Flow Perturbation Gas Chromatography”, Marcel Dekker, New York-Basel, 1988.
N. A. Katsanos, J. Chromatogr.446 39 (1988).
N. A. Katsanos, in “Theoretical Advancement in Chromatography and Related Separation Techniques”, F. Dondi, G. Guiochon, Eds., Kluwer Academic Publishers, Netherlands, 1992, p. 339.
N. A. Katsanos, Pure & Appl. Chem.65, 2245 (1993).
N. A. Katsanos, E. Dalas, J. Phys. Chem.91, 3103 (1987).
N. A. Katsanos, F. Roubani-Kalantzopoulou, J. Chromatogr. A710, 191 (1995).
V. Sotiropoulou, G. P. Vassilev, N. A. Katsanos, H. Metaxa, F. Roubani-Kalantzopoulou, J. Chem. Soc. Faraday Trans.91, 485 (1995).
R. Thede, F. Nohmie, H. Pscheidl, D. Haberland, Z. Phys. Chem.173, 87 (1991).
N. A. Katsunos, P. Agathonos, A. Niotis, J. Phys. Chem.92, 1645 (1988).
R. Atkinson, W. P. L. Carter, Chem. Rev.84, 437 (1984).
J. Troe, J. Phys. Chem.83, 114 (1979).
R. Atkinson, C. N. Plum, W. P. L. Carter, A. M. Winer, J. N. Pitts, J. Phys. Chem.88, 1210 (1984).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Sotiropoulou, V., Katsanos, N.A., Metaxa, H. et al. Gas chromatographic determination of rate constants in bimolecular gaseous reactions. Chromatographia 42, 441–450 (1996). https://doi.org/10.1007/BF02272137
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF02272137