Summary
Cilia bundled into combs or ctenes are an evolutionary innovation that allow comb jellies (animals in the phylum Ctenophora) to swim faster and grow to sizes at least two orders of magnitude larger than animals that propel themselves by beating single cilia. Ctenophore size, shape and swimming behaviors, however, may be constrained by the mechanisms that coordinate comb plate oscillations.
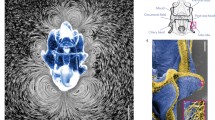
Oscillations of comb plates onPleurobrachia bachei (a cydippid comb jelly), are coupled by fluid interactions between combs. Ctenes beat metachronously (in sequence) and the flows generated byP. bachei are retarded by the amount of time it takes a wave to pass down a group of ctenes. Our model predicts thatP. bachei size is constrained by the maximum thrust that can be produced by ctenes that beat in sequence and our flow visualization studies suggest that swimming via metachronous comb oscillations may constrainP. bachei to spherical shapes.
In contrast, comb plate oscillations onMnemiopsis leidyi, a lobate comb jelly, are neurally coordinated and groups of ctenes beat in synchrony. As a result, fluid flows generated byM. leidyi are not retarded by the passage of metachronal waves down each comb row.M. leidyi reach sizes 15 times larger, but swim relatively slower (body lengths per second) thanP. bachei.
We propose that propulsion via metachronous or synchronous comb plate oscillations has played an important role in the evolution of ctenophore shape and size and may have divided comb jellies into two evolutionary lineages.
Similar content being viewed by others
References
Blake, J. R. and Sleigh, M. A. (1974) Mechanics of ciliary locomotion.Biological Review 49, 85–125.
Franc, J. M. (1978) Organization and function of ctenophore colloblasts: an ultrastructural study.Biol. Bull. mar. biol. Lab., Woods Hole155, 527–41.
Greene, C. E., Landry, M. R. and Monger, B. C. (1986) Foraging behavior and prey selection by the ambush entangling predator,Pleurobrachia bachei.Ecology 67, 1493–501.
Harbison, G. R. (1985) On the classification and evolution of the Ctenophora. InThe origins and relationships of lower invertebrates (S. C. Morris, J. D. George, R. Gibson and H. M. Platt, eds) pp. 78–100. Clarendon Press, Oxford.
Harbison, G. R. and Madin, L. P. (1982) Ctenophore. InSynopsis and classification of living organisms (S. P. Parker, ed.) pp. 707–15. McGraw-Hill, New York.
Katz, D. F. and Blake, J. R. (1974) Flagellar motions near walls. InSwimming and flying in nature. I. (T. Y.-T. Wu, C. J. Brokaw and C. Bennen, eds) pp. 173–204. Plenum Press, New York.
Knight-Jones, E. W. (1954) Relations between metachronism and the direction of ciliary beat in Metazoa.Quarterly Journal of Microscopical Science 95, 503–21.
Nachtigall, W. (1980) Mechanics of swimming in water-beatles. InAspects of Animal Movement (H. Y. Elder and E. R. Trueman, eds) pp. 107–24. Cambridge University Press, New York.
Prandt, L. and Tietjens, O. G. (1934)Applied hydro- and aeromechanics Dover Publ. New York.
Reeve, M. R. and Walter, M. A. (1978) Nutritional ecology of ctenophores—A review of recent research. InAdvances in marine biology (F. S. Russel and M. Younge, eds) pp. 249–87. Academic Press, New York.
Rouse, H. (1946)Elementary mechanics of fluids. Dover, New York.
Sleigh, M. A. and Aiello, E. (1972) The movement of water by cilia.Acta Protozoologica 11, 266–77.
Sleigh, M. A. and Barlow, D. I. (1980) Matachronism and control of locomotion in animals with many propulsive structures. InAspects of Animal Movement (A. Y. Elder and E. R. Truemon, eds) pp. 49–70. Soc. Exp. Biol. Sem. Ser. 5. Cambridge University Press, Cambridge.
Sleigh, M. A. and Blake, J. R. (1977) Methods of ciliary propulsion and their size limitations. InScale Effects of Animal Locomotion (T. J. Pedley, ed.) Academic Press.
Sleigh, M. A. and Jarman, M. (1973) Grade responses in ciliary activity of ctenophores compared with the ‘staircase’ of cardiac muscle.J. Mechanochem. Cell Motility. 2, 61–8.
Tamm, S. L. (1973) Mechanisms of ciliary coordination in ctenophores.J. Exp. Biol. 59, 231–45.
Tamm, S. L. (1982) Ctenophora. InElectrical Conduction and Behaviour in ‘Simple’ Invertebrates (G. B. Shelton, ed.) pp. 266–358. clarendon Press, Oxford.
Tamm, S. L. and Moss, A. G. (1985) Umilateral ciliary reversal and motor responses during prey capture by the ctenophorePleurobrachia.J. Exp. Biol. 114, 443–61.
Vogel, S. (1983)Life in Moving Fluids. New Jersey, Princeton.
Weihs, S. (1980) Encergetic significance of changes in swimming modes during larval anchovy,Engraulis mordax. Fisher Bulletin77, 597–604.
Wu, T. Y. (1977) Introduction to the scaling of aquatic animal locomotion. InScale Effects in Animal Locomotion (T. J. Pedley, ed.) pp. 203–32. Academic Press.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Craig, C.L., Okubo, A. Physical constrints on the evolution of ctenophore size and shape. Evol Ecol 4, 115–129 (1990). https://doi.org/10.1007/BF02270909
Issue Date:
DOI: https://doi.org/10.1007/BF02270909