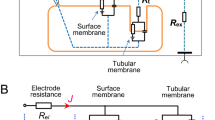
Summary
Proximal tubular cells of the frog (Rana esculenta) kidney were fused within an isolated tubule portion to giant cells according to the polyethylene-glycol fusion method. Cell membrane potentials (V m ) were measured while cells were superfused with varioús experimental solutions. Rapid concentration stepchanges of different ions allowed to calculate the respective transference numbers (t ion). In some experiments the specific cell membrane resistances (R m ) were evaluated by measuringV m induced by short current pulses injected into the cell with a second electrode. The experiments reveal: i) Fused cells of the proximal tubule exhibit aV m of −49.5±1.6 mV (n=65). ii) Addition of glucose to the perfusate yields a transient depolarization, consistent with a rheogenic Na/glucose cotransport system. iii) In absence of organic substrates the whole cell membrane conductance is made up of K+ and HCO3, iv) There is a positive relationship betweenV m andtK+ and a negative relationship betweenV m andtHCO −3 . v) HCO −3 -inducedV m changes are attenuated or abolished when Na+ is replaced with choline+, consistent with a rheogenic Na+/HCO −3 cotransport system. vi) Replacement of Na+ by choline+ depolarizesV m and increasesR m by about 50%; addition of 3 mmol/liter Ba2+ to the Na+-free perfusate increasesR m by about 58% compared to the initial control value. vii) There is no measurable cell membrane Cl− conductance. We conclude that fused cells of proximal tubule exert both luminal and peritubular membrane properties. In absence of organic substrates the cell membrane potential is determined by the HCO −3 and K+ transport systems.
Similar content being viewed by others
References
Anagnostopoulos, T., Planelles, G. 1979. Organic anion permeation at the proximal tubule ofNecturus.Pfluegers Arch. 381:231–239
Biagi, B.A., Sohtell, M. 1986. pH sensitivity of the basolateral membrane of the rabbit proximal tubule.Am. J. Physiol. 250:F261-F266
Biagi, B.A., Sohtell, M. 1986. Electrophysiology of basolateral bicarbonate transport in the rabbit proximal tubule.Am. J. Physiol. 250:F267-F272
Boron, W.F., Boulpaep, E.L. 1983. Intracellular pH regulation in the renal proximal tubule of the salamander: Basolateral HCO3 transport.J. Gen. Physiol. 81:53–94
Brazy, C.P., Dennis, V.W. 1978. Characteristics of glucosephlorizin interactions in isolated proximal tubules.Am. J. Physiol. 234:F279-F286
Grassl, S.M., Aronson, P.S. 1986. Na+/HCO3 cotransport in basolateral membrane vesicles isolated from rabbit renal cortex.J. Biol. Chem. 261:8778–8783
Guggino, W.B., Boulpaep, E.L., Giebisch, G. 1982. Electrical properties of chloride transport across theNecturus proximal tubule.J. Membrane Biol. 65:185–196
Guggino, W.B., London, R., Boulpaep, E.L., Giebisch, G. 1983. Chloride transport across the basolateral cell membrane of theNecturus proximal tubule: Dependence on bicarbonate and sodium.J. Membrane Biol. 71:227–240
Horsburgh, T., Cannon, J.K., Pitts, R.F. 1978. Action of phlorizin on luminal and antiluminal membranes of proximal cells of kidney.Am. J. Physiol. 234:F485-F489
Jentsch, T.J., Matthes, H., Keller, S.K., Wiederholt, M. 1986. Electrical properties of sodium bicarbonate symport in kidney epithelial cells (BSC-1).Am. J. Physiol. 251:F954-F968
Kawahara, K. 1985. Ba2+-sensitive potassium permeability of the apical membrane in newt kidney proximal tubule.J. Membrane Biol. 88:283–292
Kubota, T., Biagi, B.A., Giebisch, G. 1983. Effects of acid-base disturbances on the basolateral membrane potential and intracellular potassium activity in the proximal tubule ofNecturus.J. Membrane Biol. 73:61–68
Lang, F., Messner, G., Rehwald, W. 1986. Electrophysiology of sodium-coupled transport in proximal renal tubules.Am. J. Physiol. 250:F853-F962
Lang F., Oberleithner, H., Giebisch, G. 1986. Electrophysiological heterogeneity of proximal convoluted tubules inAmphiuma kidney.Am. J. Physiol. 251:F1063-F1072
Matsumura, Y., Cohen, B., Guggino, W.B., Giebisch, G. 1984. Electrical effects of potassium and bicarbonate on proximal tubule cells ofNecturus.J. Membrane Biol. 79:145–152
Matsumura, Y., Cohen, B., Guggingo, W.G., Giebisch, G. 1984. Regulation of the basolateral potassium conductance of theNecturus proximal tubule.J. Membrane Biol. 79:153–161
Messner, G., Oberleithner, H., Lang, F. 1985. The effect of phenylalanine on the electrical properties of proximal tubule cells in the frog kidney.Pfluegers Arch. 404:138–144
Messner, G., Wang, W., Paulmichl, M., Oberleithner, H., Lang, F. 1985. Ouabain decreases apparent potassium-conductance in proximal tubules of the amphibian kidney.Pfluegers Arch. 404:131–137
Oberleithner, H., Gassner, B., Dietl, P., Wang, W. 1986. Amphibian nephron: Isolated kidney and cell fusion.Methods Enzymol. (in press)
Oberleithner, H., Schmidt, B., Dietl, P. 1986. Fusion of renal epithelial cells: A model for studying cellular mechanisms of ion transport.Proc. Natl. Acad. Sci. USA 83:3547–3591
Planelles, G., Teulon, J., Anagnostopoulos, T. 1981. The effects of barium on the electrical properties of the basolateral membrane in proximal tubule.Naunyn-Schmiedeberg's Arch. Pharmacol. 318:135–141
Sackin, H., Boulpaep, E.L. 1981. Isolated perfused salamander proximal tubule. II. Monovalent ion replacement and rheogenic transport.Am. J. Physiol. 241:F540-F555
Sakhrani, L.M., Badie-Dezfooly, B., Trizna, W., Mikhail, N., Lowe, A.G., Taub, M., Fine, L.G. 1984. Transport and metabolism of glucose by renal proximal tubular cells in primary culture.Am. J. Physiol. 246:F757-F764
Wang, W., Dietl, P., Oberleithner, H. 1987. Evidence for Na+ dependent rheogenic HCO −3 transport in fused cells of frog distal tubules.Pfluegers Arch. 408:291–299
Westerwoudt, R.J. 1985. Improved fusion methods. IV. Technical aspects.J. Immunol. Meth. 77:181–196
Wojcieszyn, J.W., Schlegel, R.A., Lumley-Sapansky, K., Jacobson, K.A. 1983. Studies on the mechanism of polyethylene glycol-mediated cell fusion using fluorescent membrane and cytoplasmic probes.J. Cell Biol. 96:151–159
Yoshitomi, K., Burckhardt, B.C., Frömter, E. 1985. Rheogenic sodium-bicarbonate cotransport in the peritubular cell membrane of rat renal proximal tubule.Pfluegers Arch. 405:360–366
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Dietl, P., Wang, W. & Oberleithner, H. Fused cells of frog proximal tubule: I. Basic membrane properties. J. Membrain Biol. 100, 43–51 (1987). https://doi.org/10.1007/BF02209139
Received:
Revised:
Issue Date:
DOI: https://doi.org/10.1007/BF02209139