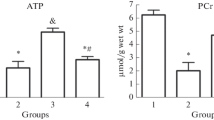
Summary
The left descending coronary artery (LAD) was ligated for 45 min or 90 min followed by 1-h reperfusion. During experiments the animals in Group I (15 dogs) received saline infusion, in Group II (20 dogs) they received the ultrashort-acting β-blocker Brevibloc (esmolol HCl). The marker of lipid peroxidation the malondialdehyde (MDH) as well as endogen scavengers, the glutathione (GSH), and superoxide dismutase (SOD) were measured in the heart tissue homogenates. In blood and heart tissue samples the 6-keto-prostaglandin F1 α. (PGF1 α) and thromboxane B2 (TXB2) were determined. Biochemical measurements revealed that esmolol HCl has beneficial effect on the free-radical-meduated-damage reducing the MDA content in the ischemic area. In Group I the value of MDA after 90 min of LAD ligature was 138 ± 5.6 %, in Group II the elevation was only 107.4 ± 3.2 %. After treatment with Brevibloc the GSH content of ischemic-reperfused areas decreased slightly (81.75 ± 3.5 % of the normal value), moreover, in Group I the depletion of GSH was considerable (64.5 ± 4.2 %). Coronary reperfusion caused the release of eicosanoids in both groups, mainly in the first 10 min. The highest value of thromboxane in blood samples could be measured in Group I after 90 min of LAD ligature (24.8 ± 3.6 pmol/ml; the normal value 8–12 pmol/ml). In Group II during the same period of experiments the TXB2 in the blood was 14 ± 3.7 pmol/ml. In heart tissue samples the amount of endoperoxides increased in ischemic and non-ischemic areas of Group I and II. Nevertheless, the calculated ratio of PGF1 α and TXB2 was near to the normal after Brevibloc treatment (0.85–0.9; the normal values were 1–1.2). These results indicate that esmolol HCl can modulate both the free-radical-mediated reaction and arachidonic acid metabolism.
Similar content being viewed by others
Abbreviations
- 6-keto-PGF1α :
-
6-keto-prostaglandin F1α
- TXB2 :
-
thromboxane B2
- MDA:
-
malondialdehyde
- GSH:
-
reduced glutathione
- SOD:
-
superoxid dismutase
- CPK:
-
creatine kinase
- LAD:
-
left descending coronary artery
References
Bache RJ (1984) Can drugs really limit infarct size? In: Hearse DJ, Yellon DM (eds) Therapeutic approaches to myocardial infarct size limitation. Raven Press, New York, pp 185–208
Braunwald E, Kloner RA (1985) Myocardial reperfusion: A double-edged sword. J Clin Invest 76:1713–1719
Burton KP, Buja LM, Sen A (1986) Accumulation of arachidonate in triacylglycerols and unesterified fatty acids during ischemia and reflow in the isolated rat heart. Correlation with loss of contractile function and the development of calcium overload. Am J Pathol 124:238–245
Campbell WB, Johnson AR, Callahan KS, Graham RM (1981) Anti-platelet activity of betaadrenergic antagonists: Inhibition of thromboxane synthesis and platelet aggregation in patients receiving long-term propranolol treatment. The Lancet, December 19/26:1382–1384
Campbell CA, Przyklenk K, Kloner RA (1986) Infarct size reduction: A review of the clinical trials. J Clin Pharmacol 26:317–329
Carpenter MP (1981) Antioxidant effects on the prostaglandin endoperoxide synthetase product profile. Fed Proc 40:189–194
Chan PS, Cervoni P (1986) Prostaglandins, prostacyclin and thromboxane in cardiovascular diseases. Drug Development Res 7:341–359
Coker SJ, Parratt JR, Ledingham I MCA, Zeitlin IJ (1981) Thromboxane and prostacyclin release from ischemic myocardium in relation to arrhythmias. Nature 291, 28 May
Covinsky JO (1987) Esmolol: A novel cardioselective, titratable, intravenous beta-blocker with ultrashort half-life. Drug Intell Clin Pharm 21:316–321
Farber JL, Chien KR, Mittnacht S (1981) The pathogenesis of irreversible cell injury in ischemia. Am J Pathol 10:271–281
Ferrari R, Ceconi C, Curello S et al (1985) Oxygen-mediated myocardial damage during ischemia and reperfusion: Role of the cellular defenses against oxygen toxicity. J Mol Cell Cardiol 17:937–945
Fiedler VB (1988) Role of arachidonic acid metabolites in cardiac ischemia and reperfusion injury. Pharmacotherapy 8(3):158–168
Frishman WH (1988) Beta-adrenergic blockers. Medical Clinics of North America Vol 72, No 1:37–81
Frishman WH, Skolnick AE, Lazar EJ, Fein S (1989) Beta-adrenergic blockade and calcium channel blockade in myocardial infarction. Medical Clinics of North America. Vol 73, No 2:409–436
Gorczynski RJ, Shaffer JE, Lee RJ (1983) Pharmacology of ASL-8052, a novel beta-adrenergic receptor antagonist with an ultrashort duration of action. J Cardiovasc Pharmacol 5:668–677
Guarnieri C, Flamingi F, Caldarera CM (1980) Role of oxygen in the cellular damage induced by re-oxygenation of hypoxic heart. J Mol Cell Cardiol 12:797–808
Guarnieri C, Muscari C, Fraticelli A, Caldarera CM (1988) Role of antioxidants in hypoxiareoxygenation injury in the heart and in cardiac myocytes. In: Signal PK (ed) Oxygen radicals in the pathophysiology of heart disease. Kluwe Academic Publishers, Boston Dordrecht Lancaster, pp 271–283
Hess ML, Manson NH (1984) Molecular oxygen: friend and foe. The role of oxygen free radical system in the calcium paradox, the oxygen paradox and ischemic/reperfusion injury. J Mol Cell Cardiol 16:969–985
Jennings RR, Reimer KA (1979) Effect of beta-adrenergic blockade on acute myocardial ischemic injury. In: Gross F (ed) Modulation of sympathetic tone in the treatment of cardiovascular diseases. Hans Huber, Berne, pp 103–114
Jennings RB, Reimer KA (1983) Factors involved in salvaging ischemic myocardium: Effect of reperfusion of arterial blood. Circulation 68:I-25–I-36
Johnson AR, Campbell WB (1980) Antiplatelet activity of Propranolol: Inhibition of thromboxane synthetase. Fed Proc 39:3920
Jurgensen HJ, Frederiksen J, Hansen DA, Pedersen-Bjergaard O (1981) Limitation of myocardial infarct size in patients less than 66 years treated with alprenolol. Br Heart J 45:583–588
Kirshenbaum JM, Kloner RA, Amman EM, Braunwald E (1985) Use of an ultra short-acting betablocker in patients with acute myocardial ischemia. Circulation 72:873–880
Kloner RA, Kirshenbaum J, Lange R, Antman EM, Braunwald E (1985) Experimental and clinical observations on the efficacy of esmolol in myocardial ischemia. Am J Cardiol 56:40F-48F
Kloner RA (1988) Introduction to the role of oxygen radicals in myocardial ischemia and infarction. Free Radic Biol Med 4(1):5–7
Lange R, Kloner RA, Braunwald E (1983) First ultra-short-acting beta-adrenergic blocking agent: its effect on size and segmental wall dynamics of reperfused myocardial infarcts in dog. Am J Cardiol 51:1759–1767
Lefer AM (1985) Eicosanoids as mediators of ischemia and shock. Fed Proc 44:275–280
Maza SR, Frishman WH (1988) Therapeutic options to minimize free radical damage and thrombogenicity in ischemic/reperfused myocardium. Med Clin North Am 72:227–242
McCord JM (1985) Oxygen-derived free radicals in postischemic tissue injury. N Engl J Med 312:159–163
Misra HP, Fridovich I (1972) The role of superoxide anion in the antioxidation of epinephrine and a simple assay for superoxide dismutase. J Biol Chem 247:3170–3175
Parratt JR, Coker SJ, Wainwright CHL (1987) Eicosanoids and susceptibility to ventricular arrhythmias during myocardial ischemia and reperfusion. J Mol Cell Cardiol 19(Suppl V):55–66
Ross D (1988) Glutathione, free radicals and chemotherapeutic agents. Pharmac Ther 37:231–249
Röth E, Török B, Pollák ZS, Temes GY, Morvay G (1987) Myocardial protection by antioxidant during permanent and temporary coronary occlusion in dogs. In: Jacob R, Just HJ, Holubarsch Ch (eds) Cardiac energetics. Steinkopff, Darmstadt; Springer, New York, pp 335–345
Röth E, Kelemen D, Török B, Nagy A, Pollak S (1989) Dynamics of prostacyclin and thromboxane during myocardial ischemia. Prog Clin Biol Res 308:907–911
Sum CY, Yacobi A, Kartzinel R, Stampfil H, Davis CS, Lai CM (1983) Kinetics of esmolol, an ultra-short-acting beta blocker, and of its major metabolite. Clin Pharmacol Ther 34:427–434
Sung J, Blanski L, Kirsbenbaum J, MacCosbe P, Turlapaty P, Laddu AR, and the Esmolol Research Group (1986) Clinical experience with esmolol, a short-acting beta-adrenergic blocker in cardiac arrhythmias and myocardial ischemia. Clin Pharmacol 26:A15-A26
The Esmolol Multicenter Study Research Group (1985) Efficacy and safety of esmolol vs propranolol in the treatment of supraventricular tachyarrhythmias: A multicenter double-blind clinical trial. Am Heart J 5:913–921
Tietze F (1969) Enzymic method for quantitative determination of nanogram amounts of total and oxidized glutathione: applications to mammalian blood and other tissues. Anal Biochem 27:502–522
Torr S, Drake-Holland AJ, Main M, Hynd J, Isted K, Noble MIM (1989) Effects on infarct size of reperfusion and pretreatment with beta-blockade and calcium antagonists. Basic Res Cardiol 84:564–582
Török B, Röth E, Bar V, Pollák ZS (1986) Effects of antioxidant therapy in experimentally induced heart infarcts. Basic Res Cardiol 81:167–179
Török B, Röth E, Mezey B, Temes GY, Tóth K, Pollák ZS (1987) Promising reduction of ventricular fibrillation in experimentally induced heart infarction by antioxidant therapy. In: Jacob R, Just HJ, Holubarsch CH (eds) Cardiac energetics. Steinkopff, Darmstadt; Springer, New York, pp 347–353
Zaroslinski J, Borgman RJ, O'Donnell JP, Anderson WC, Erhardt PW, Kam ST, Reynolds RD, Lee RJ, Gorczynski RJ (1982) Ultra-short acting beta-blockers: A proposal for the treatment of the critically ill patient. Life Sciences 31:899–907
Yusuf S, Sleight P, Rossi P, Ramsdale D, Peto R, Furze L, Sterry H, Pearson M, Motwani R, Parish S, Gray R, Bennett D, Bray C (1983) Reduction in infarct size, arrhythmias and chest pain by early intravenous beta-blockade in suspected acute myocardial infarction. Circulation 67:I-32–I-41
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Röth, E., Török, B. Effect of the ultrashort-acting β-blocker Brevibloc on free-radical-mediated injuries during the early reperfusion state. Basic Res Cardiol 86, 422–433 (1991). https://doi.org/10.1007/BF02190710
Received:
Issue Date:
DOI: https://doi.org/10.1007/BF02190710