Abstract
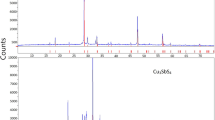
The reaction process between CuSO4 and excess Cu2S in the temperature range 650–750 K was investigated by methods of thermal analysis and by studying the phase contentss of the products as a function of the fractional conversion. The reaction proceeds in three steps, with Cu2S and a new phase described by the formula Cu2SO2 as intermediates. This new phase is liquid under the conditions of the reaction. The final product of the reaction is a defective crystalline Cu2O.
Zusammenfassung
Der Verlauf der Reaktion zwischen CuSO4 und überschüssigem Cu2S im Temperaturbereich von 650–750 K wurde mittels thermoanalytischer Methoden und durch Ermittlung der Phasenzusammensetzung in AbhÄngigkeit von der Konversion untersucht. Die Reaktion verlÄuft in drei Schritten mit Cu2S und einer neuen Phase der Zusammensetzung Cu2SO2 als Zwischenproduke. Die neue Phase ist unter den Reaktionsbedingungen eine Flüssigkeit. Endprodukt der Reaktion ist nicht völlig kristallines Cu2O.
РЕжУМЕ
РЕАкцИОННОИ пРОцЕсс МЕжДУ CuSO4 И ИжБыткОМ Cu2S Был ИсслЕДОВАН В ОБлА стИ тЕМпЕРАтУР 650–750 K МЕтОДО М тЕРМИЧЕскОгО АНАлИ жА И ИжУЧЕНИЕМ ФАжОВОгО с ОстАВА пРОДУктОВ В жАВИсИМО стИ От ФРАкцИОНИРОВА ННОгО пРЕВРАЩЕНИь. РЕАкцИь пРОтЕкАЕт В тРИ стАДИИ с ОБРАжОВАНИЕ М пРОМЕжУтОЧНых пРОД УктОВ Cu2S И НОВОИ ФАжы, ОпИсыВАЕМ ОИ ФОРМУлОИ Cu2SO2 И кОтОРАь В УслОВИь х РЕАкцИИ ьВльЕтсь жИ ДкОИ. кОНЕЧНыМ пРОДУктОМ РЕАкцИИ ьВльЕтсь кРИ стАллИЧЕскАь Cu2O с НАРУ шЕННОИ стРУктУРОИ.
Similar content being viewed by others
References
J. Walczak, Prace Nauk, Pol. Szczec., nr 50, (1975).
T. R. Ingraham, Trans. Met. Soc. AIME, 233 (1965) 359.
W. Reinders and F. Goudrian, Z. Anorg. Allg. Chem., (1923), 85.
R. Schenck and E. Hempelmann, Z. Angew. Chem., 26 (1913), 685.
J. V. Margulis and V. D. Ponomarev, Izv. Akad. Nauk., Ser. met. obog. i ogneup., 3 (1958) 9.
J. V. Margulis, Sb. Nautch. Tr. Vses. Nautch.-Issled. Gornomet. Inst. Cvet. Met., 17 (1968) 5.
M. Nagamori and F. Habashi, Met. Trans., 5 (1974) 523.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Dębiński, H., Walczak, J. Studies of the reaction mechanism between copper(II) sulphate and excess copper(I) sulphide. Journal of Thermal Analysis 29, 977–981 (1984). https://doi.org/10.1007/BF02188846
Issue Date:
DOI: https://doi.org/10.1007/BF02188846