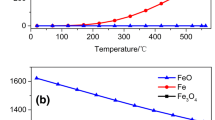
Abstract
The conditions for the direct carbothermic reduction of metals from mixtures of sulfides Cu1.96S and FeS with calcium oxide are studied using thermogravimetric and thermal analyses combined with mass spectrometry of the formed gases. The metal reduction in a mixture of FeS and CaO on heating to 1250°C is shown to occur with the formation of intermediate oxysulfide phases almost without the evolution of sulfur-containing gases. The formation of SO2 is observed in a mixture of Cu1.96S and CaO at temperatures higher than 550°C. The introduction of FeS into the reaction mixture of Cu1.96S and CaO enhances the completeness of metal reduction by carbon, decreases the temperatures of the intense processes, and suppresses sulfur dioxide evolution.






Similar content being viewed by others
REFERENCES
L. N. Ertseva, V. T. D’yachenko, and S. V. Sukharev, “Reductive thermal treatment of pyrrhotine from the pyrrhotine-containing copper–nickel sulfide raw materials,” Tsvetn. Met., No. 5, 18–21 (1997).
L. N. Ertseva, V. T. D’yachenko, and S. V. Sukharev, “Reductive thermal treatment of chalcopyrite from the pyrrhotine-containing copper–nickel sulfide raw materials,” Tsvetn. Met., No. 9, 11–13 (1997).
A. Jha and P. Grieveson, “Carbothermic reduction of pyrrhotite in the presence of lime for production of metallic iron. II. Kinetics and mechanism of reduction,” Scand. J. Met. 21, 50–61 (1992).
A. Jha, U. O. Igiehon, and P. Grieveson, “Carbothermic reduction of pyrrhotite in the presence of lime for production of metallic iron. I. Phase equilibria in Fe–Ca–S–O system,” Scand. J. Met. 20, 270–278 (1991).
N. Machingawuta, A. Jha, and P. Grieveson, “Mechanism of carbothermic reduction of nickel sulfide mineral in the presence of lime,” Scand. J. Met. 18, 81–88 (1989).
A. Jha, P. Grieveson, and J. H. E. Jeffes, “An investigation on the carbothermic reduction of nickel sulfide minerals: Kinetic and thermodynamic consideration,” Scand. J. Met. 18, 31–45 (1989).
M. Moinpour and Y. K. Rao, “Direct reduction of copper sulphide with carbon in the presence of lime,” Can. Met. Quart. 24 (1), 69–81 (1985).
NETZSCH Thermokinetics 3.0. Version 2006.08. www.therm-soft.com.
“Powder diffraction file-2 (PDF-2+),” in International Centre for Diffraction Data (ICCD) (2012).
Outokumpu HSC Chemistry for Windows. Chemical Reaction and Equilibrium Software with Extensive Thermochemical Database HSC. Version 6.2.8.
G. V. Novikov, V. K. Egorov, and Yu. A. Sokolov, Pyrrhotines (Nauka, Moscow, 1988).
V. I. Yarygin, N. I. Kopylov, V. N. Novoselova, et al., “Influence of calcium oxide on the fusibility of iron, copper, zinc, and lead sulfides,” Izv. Akad. Nauk SSSR, Met., No. 6, 65–68 (1975).
E. N. Selivanov, V. M. Chumarev, R. I. Gulyaeva, V. P. Mar’evich, A. D. Vershinin, A. A. Pankratov, and E. S. Korepanova, “Composition, properties, and thermal expansion of iron–calcium oxysulfides,” Neorg. Mater. 40 (8), 969–974 (2004).
O. A. Esin and P. V. Gel’d, Physical Chemistry of Pyrometallurgical Processes (Metallurgizdat, Moscow, 1950), Part I.
V. M. Chumarev, A. I. Okunev, and R. I. Shakirzyanova, “Reduction of iron–calcium oxysulfide by carbon and carbon oxide,” Izv. Akad. Nauk SSSR, Met., No. 3, 10–15 (1977).
R. Blachnik and A. Muller, “The formation of Cu2S from the elements. 1. Copper used in form of powders,” Thermochim. Acta 361, 31–52 (2000).
N. I. Kopylov, M. P. Smirnov, and M. Z. Toguzov, Phase Diagrams in Metallurgy of Heavy Nonferrous Metals (Metallurgiya, Moscow, 1993).
V. P. Mar’evich, R. I. Gulyaeva, E. N. Selivanov, V. M. Chumarev, and A. A. Pankratov, “On the new phase of copper-containing iron–calcium oxysulfide,” in Proceedings of XV International Conference on Chemical Thermodynamics in Russia (Mosk. Gos. Univ., Moscow, 2005), Vol. 2, p. 225.
D. O. Charkin, A. V. Sadakov, O. E. Omel’yanovskii, and S. M. Kazakov, “Synthesis, crystal structure, and properties of novel perovskite oxychalcogenides, Ca2CuFeO3Ch (Ch = S, Se),” Mater. Res. Bull. 45, 2012–2016 (2010).
R. Gulyaeva, E. Selivanov, and A. Mansurova, “Kinetics of the calcium oxysulfides reduction by carbon monoxide,” Defect Diffusion Forum 283–286, 539–544 (2009).
J. Opfermann, “Kinetic analysis using multivariate non-linear regression. I. Basic concepts,” J. Therm. Anal. Calorim. 60, 641–658 (2000).
E. T. Turkdogan, Physical Chemistry of High Temperature Technology (Academic, New York, 1980).
ACKNOWLEDGMENTS
This work was carried out in terms of state assignment for the Institute of Metallurgy (Ural Branch, Russian Academy of Sciences) using the equipment of the Center for Collective Use Ural-M.
Author information
Authors and Affiliations
Corresponding author
Additional information
Translated by E. Yablonskaya
Rights and permissions
About this article
Cite this article
Selivanov, E.N., Gulyaeva, R.I. Carbothermic Reduction of Metals in the FeS–Cu1.96S–CaO System. Russ. Metall. 2019, 216–222 (2019). https://doi.org/10.1134/S003602951903011X
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S003602951903011X