Abstract
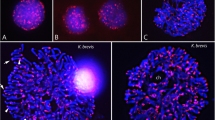
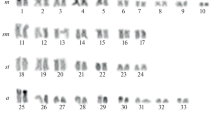
DNA reassociation kinetics were used to determine nuclear genome organization and complexity inGymnogongrus griffithsiae (Gigartinales, Rhodophyta). Results indicate the presence of three second order components corresponding to fast (3%), intermediate (8%) and slow (89%) fractions. Thus the genome consists mainly of unique sequences. Thermal denaturation (T m) indicated a nuclear DNA base pair composition of 40 mol% G + C. Microspectrophotometry with the DNA-localizing fluorochrome DAPI was used to confirm ploidy level differences in the gametophytic and tetrasporoblastic phases. Comparisons of mean nuclear DNA (I f) values to chicken erythrocytes (RBC) resulted in an estimate of 0.32 pg/2 C genome forGymnogongrus griffithsiae. Karyological studies using aceto-orcein revealed the presence of ca. 23 bivalents during diakinesis of tetrasporangial mother cells. Total carrageenan content in water extraction was 30% dry weight. Infrared spectroscopy confirmed the isolated carrageenan to be the iota-fraction.
Similar content being viewed by others
References
Abbot I, Cheney D (1982) Commercial uses of algal products: introduction and bibliography. In Rosowski JR, Parker BC (eds), Selected Papers in Phycology II. Phycol. Soc. Amer., Lawrence, Kansas, 779–787.
Britten RJ, Davidson EH (1971) Repetitive and nonrepetitive DNA sequences: a speculation on the evolutionary novelty. Q. Rev. Biol. 46: 11–133.
Britten RJ, Kohne DE (1970) Repeated segments of DNA. Sci. Am. 222: 24–31.
Clowes AW, Reidy MA, Clowes MM (1983) Kinetics of cellular proliferation after arterial injury. I. Smooth muscle growth in the absence of endothelium. Lab. Investigations 49: 327–333.
Cole KM (1990) Chromosomes. In Cole KM, Sheath RG (eds), Biology of the Red Algae. Cambridge University Press, New York, 73–101.
Cordeiro-Marino M, Poza AC (1981) Life history ofGymnogongrus griffithsiae (Turner) Martius (Phyllophoraceae, Gigartinales). Proc. Int. Seaweed Symp. 10: 155–161.
Craigie JS (1990) Cell walls. In Cole KM, Sheath RG (eds), Biology of the Red Algae. Cambridge University Press, New York, 221–258.
DeCew TC, West JA (1981) Life histories in the Phyllophoraceae (Rhodophyta:Gigartinales) from the Pacific Coast of North America. I.Gymnogongrus linearis andG. leptophyllus. J. Phycol. 17: 240–250.
Doubt DG (1935) Notes on two species ofGymnogongrus. Am. J. Bot. 22: 294–310.
Dutcher JA, Sizemore RK, Kapraun DF (1990a) Variation in nuclear DNA base composition (Mol% G + C) in three genera of Gigartinales (Rhodophyta). J. Cryptogam. Bot. 1: 390–395.
Dutcher JA, Kapraun DF, Sizemore RK (1990b) Inter- and intraspecific variation of nuclear DNA reassociation kinetics in the Gracilariales (Rhodophyta). J. appl. Phycol. 2: 259–267.
Fuller SW, Mathieson AC (1972) Ecological studies of economic red algae. IV. Variations of carrageenan concentration and properties inChondrus crispus Stackhouse. J. exp. mar. Biol. Ecol. 10: 49–58.
Freshwater DW, Dutcher JA, Kapraun DF, Sizemore RK (1990) Variation in nuclear DNA base composition (mol% G + C) in three orders of marine green algae. In Lindstrom SC, Gabrielson PW (eds), Thirteenth International Seaweed Symposium. Developments in Hydrobiology. 58. Kluwer Academic Publishers, Dordrecht, 167–172. Reprinted from Hydrobiologia 204/205.
Glicksman PM (1987) Utilization of seaweed hydrocolloids in the food industry. In Ragan MA, Bird CJ (eds), Twelfth International Seaweed Symposium. Developments in Hydrobiology 41. Dr W. Junk Publishers, Dordrecht, 31–47. Reprinted from Hydrobiologia 151/152.
Golf LJ, Coleman AW (1984) Elucidation of fertilization and development in a red alga by quantitative DNA microspectrofluorometry. Devel. Biol. 102, 173–194.
Goff LJ, Coleman AW (1985) The role of secondary pit connections in red algal parasitism. J. Phycol. 21: 483–508.
Goff LJ, Coleman AW (1986) A novel pattern of apical cell polyploidy, sequential polyploidy reduction and intercellular nuclear transfer in the red algaPolysiphonia. Am. J. Bot. 73: 1109–1130.
Goff LJ, Coleman AW (1990) DNA microspectrofluorometry. In Cole KM, Sheath RG (eds), Biology of the Red Algae. Cambridge University Press, New York, 43–72.
Guiry MD, Garbary D (1990) A preliminary phylogenetic analysis of the Phyllophoraceae, Gigartinaceae and Petrocelidaceae (Rhodophyta) in the North Atlantic and North Pacific. In Garbary DJ, South GR (eds), Evolutionary Biogeography of the Marine Algae of the North Atlantic. Springer-Verlag, Berlin, 241–264.
Johnson OW, Utter FM, Rabinovitch PS (1987) Differences in salmonid cellular DNA identified by flow cytometry. Copeia 4: 1001–1009.
Kapraun DF (1980) An Illustrated Guide to the Marine Algae of Coastal North Carolina I. Rhodophyta. University of North Carolina Press, Chapel Hill, 206.
Kapraun DF (1989) Karyological investigations of chromosome variation patterns associated with speciation in some Rhodophyta. In George RY, Hulbert AW (eds), Carolina Coastal Oceanography Symposium. NOAA-NURC Report 89(2): 65–76
Kapraun DF, Dutcher JA (1991) Cytophotometric estimation of inter- and intraspecific nuclear DNA content inGracilaria andGracilariopsis (Gracilariales, Rhodophyta). Bot. Mar. 34: 139–144.
Kapraun DF, Hinson TK, Lemus AJ (1991) Karyology and cytophotometric estimation of inter- and intraspecific nuclear DNA variation in four species ofPorphyra (Rhodophyta). Phycologia 30: 458–466.
Kapraun DF, Dutcher JA, Lopez-Bautista JC (1992) Nuclear genome characterization of the carrageenophyteAgardhiella subulata (Rhodophyta). J. appl. Phycol. 4: 1–9.
Kurabayashi M, Lewis H, Raven PH (1962) A comparative study of mitosis in the Onagraceae. Am. J. Bot. 49: 1033–1086.
Lewin BD (1990) A continuum of sequences includes structural genes. In Genes IV. Oxford University Press, New York, 857.
Lewis NI, Avila M, McLachlan JL (1991) Life history ofGymnogongrus furcellatus (C. Ag.) J. Ag. (Rhodophyta, Phyllophoraceae) from Chile. Bot. Mar. 34: 145–152.
Maggs CA (1988) Intraspecific life history variability in the Florideophycidae (Rhodophyta). Bot. Mar. 31: 465–490.
Maggs CA (1989)Erythrodermis allenii Batters in the life history ofPhyllophora traillii Holmes ex Batters (Phyllophoraceae, Rhodophyta). Phycologia 28: 305–317.
Maggs CA (1990) Taxonomy of phyllophoroid algae: the implications of life history. In Lindstrom SC, Gabrielson PW (eds), Thirteenth International Seaweed Symposium. Developments in Hydrobiology 58. Kluwer Academic Publishers, Dordrecht, 119–124. Reprinted from Hydrobiologia 204/205.
Maggs CA, Pueschel CM (1989) Morphology and development ofAhnfeltia plicata (Rhodophyta): proposal of Ahnfeltiales ord. nov. J. Physol. 25: 333–251.
Maggs CA, Douglas SE, Fenety J, Bird CJ (1992) A molecular and morphological analysis of theGymnogongrus devoniensis (Rhodophyta, Phyllophoraceae) complex in the North Atlantic. J. Phycol. 28: 214–232.
Marmur J, Falkow S, Mandel M (1963) New approaches to bacterial taxonomy. Annu. Rev. Microbiol. 17: 329–372.
Mathieson AC, Tveter E (1975) Carrageenan ecology ofGigartina stellata (Stackhouse) Batters. Aquatic Botany: 2: 353–361.
McCandless EL, Gretz MR (1984) Biochemical and immunological analysis of carrageenans of the Gigartinaceae and Phyllophoraceae. In Bird CJ, Ragan MA (eds), Eleventh International Seaweed Symposium. Developments in Hydrobiology 22. Dr W. Junk Publishers, Dordrecht, 175–178. Reprinted from Hydrobiologia 116/117.
McCandless EL, West JA, Guiry MD (1983) Carrageenan patterns in the Phyllophoraceae. Biochem. Syst. Ecol. 10: 275–284.
McHugh DJ (1984) Marine phycoculture and its impact on the seaweed colloid industry. In Bird CJ, Ragan MA (eds), Eleventh International Seaweed Symposium. Developments in Hydrobiology 22. Dr W. Junk Publishers, Dordrecht, 351–354. Reprinted from Hydrobiologia 116/117.
Natarajan AT, Ahstrom G (1969) Heterochromatin and chromosome aberrations. Chromosoma (Berl.) 28: 48–61.
Newroth PR (1972) Studies on the life histories in the Phyllophoraceae. II.Phyllophora pseudoceranoides and notes onP. crispa andP. heredia (Rhodophyta, Gigartinales). Phycologia 11: 99–107.
Pakhomova MV, Zajceva GN, Belozerskij AN (1968) Presence of 5-methylcytosine and 6-methylaminopurine in the DNA of some algae. Dokl. Akad. Nauk. SSR. 182: 712–714.
Parsons TJ, Maggs CA, Douglas SE (1990) Plastid restriction analysis links the heteromorphic phases of an apomictic red algal life history. J. Phycol. 26: 495–500.
Rochas C, Lahaye M, Yaphe W (1986) Sulfate content of carrageenan and agar determined from infrared spectroscopy. Bot. Mar. 29: 335–340.
Saito RM, Oliveira EC (1990) Chemical screening of Brazilian marine algae producing carrageenans. In Lindstrom SC, Gabrielson PW (eds), Thirteenth International Seaweed Symposium. Developments in Hydrobiology 58. Kluwer Academic Publishers, Dordrecht, 585–588. Reprinted from Hydrobiologia 204/205.
Stanicoff DJ, Stanley NF (1969) Infrared and chemical studies on algal polysaccharides. In Margaleff R (ed.), Proc. Intl. Seaweed Symp. 6: 595–609.
Tiersch TR, Chandler RW, Kallman KD, Wachtel SS (1989) Estimation of nuclear DNA content by flow cytometry in fishes of the genusXiphophorus. Comp. Biochem. Physiol. 94B: 465–468.
Westbrook MA (1928) Observations on nuclear structure in the Florideae. Beih. Bot. Centrabl. 53A: 564–585
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Kapraun, D.F., Dutcher, J.A., Bird, K.T. et al. Nuclear genome characterization and carrageenan analysis ofGymnogongrus griffithsiae (Rhodophyta) from North Carolina. J Appl Phycol 5, 99–107 (1993). https://doi.org/10.1007/BF02182427
Received:
Revised:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF02182427