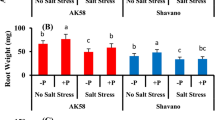
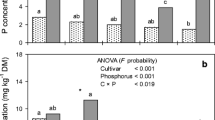
Summary
Ginger, cassava, maize, wheat, french bean and tomato were grown for periods up to six weeks in continuously flowing nutrient solutions at seven constant pH values ranging from 3.3 to 8.5. All species achieved maximum or near-maximum growth in the pH range 5.5 to 6.5. However, there were substantial differences in the ability of species to grow outside this range. Ginger and cassava were the most tolerant species to low solution pH, while ginger and tomato were the only species to show no yield depression at the highest solution pH. Roots of all species at pH 3.3 and some species at pH 4.0 exhibited symptoms of hydrogen ion injury. In addition, the concentrations of magnesium in the tops of all six species, of nitrogen in the tops of tomato and cassava, and of manganese in the tops of maize at these pH values were inadequate for optimal growth. Growth depression at high solution pH was associated with iron deficiency in maize and wheat and with nitrogen and/or copper deficiency in cassava.
The relevance of the present results to crop growth under field conditions is discussed. The complex interplay of plant and soil characteristics militates against precise definition of an optimum pH range for the growth of a particular crop unless the soil is also specified.
Similar content being viewed by others
References
Andrew, C. S. 1976 Effect of calcium, pH and nitrogen on the growth and chemical composition of some tropical and temperature pasture legumes. I. Nodulation and growth. Aust. J. Agric. Res.27, 611–623.
Andrew, C. S. and Johnson, A. D. 1976 Effect of calcium, pH and nitrogen on the growth and chemical composition of some tropical and temperate pasture legumes. II. Chemical composition (calcium, nitrogen, potassium, magnesium, sodium and phosphorus). Aust. J. Agric. Res.27, 625–636.
Arnon, D. I. and Johnson, C. M. 1942 Influence of hydrogen ion concentration on the growth of higher plants under controlled conditions. Plant Physiol.17, 525–539.
Arnon, D. I., Fratzke, W. E. and Johnson, C. M. 1942 Hydrogen ion concentration in relation to absorption of inorganic nutrients by higher plants. Plant Physiol.17, 515–524.
Asher, C. J. and Edwards, D. G. 1978 Relevance of dilute solution culture studies to problems of low fertility tropical soils,In Mineral Nutrition of Legumes in tropical and subtropical Soils. Eds. C. S. Andrew and E. J. Kamprath pp. 131–152. CSIRO, Melbourne, Australia.
Asher, C. J. and Lee, M. T. 1975 Diagnosis and correction of nutritional disorders in ginger. Department of Agriculture, University of Queensland, Brisbane, Australia.
Asher, C. J., Ozanne, P. G. and Loneragan, J. F. 1965 A method for controlling the ionic environment of plant roots. Soil Sci.100, 149–156.
Bassioni, N. H. 1971 Temperature and pH interaction in nitrate uptake. Plant and Soil35, 445–448.
Bowen, J. E. 1969 Absorption of copper, zinc and manganese by sugarcane leaf tissue. Plant Physiol.44, 255–261.
Chaudry, F. M. and Loneragan, J. F. 1972 Zinc absorption by wheat seedlings. II. Inhibition by hydrogen ions and by micronutrient cations. Soil Sci. Soc. Am. Proc.36, 327–331.
CIAT, Cali, Colombia Annu. Rep. (1974).
CIAT, Cali, Colombia Annu. Rep. (1977).
Edwards, D. G. and Asher, C. J. 1974 The significance of solution flow rate in flowing culture experiments. Plant and Soil41, 161–175.
Edwards, D. G. and Kang, B. T. 1978 Tolerance of cassava (Manihot esculenta Crantz) to high soil acidity. Field Crops Res.1, 337–346.
Epstein, E. 1972 Mineral Nutrition of Plants: Principles and Perspectives, John Wiley and Sons, Inc., New York, 412 p.
Forno, D. A. 1977 The mineral nutrition of cassava (Manihot esculenta Crantz) with particular reference to nitrogen. Doctoral Thesis, University of Queensland, Brisbane, Australia.
Forno, D. A., Asher, C. J. and Edwards, D. G. 1976 Mist propagation of cassava tip cuttings for nutritional studies: effects of substrate calcium concentration, temperature and shading. Trop. Agric. Trinidad53, 47–55.
Fox, R. H., Talleyrand, H. and Scott, T. W. 1975 Effect of nitrogen fertilization on yields and nitrogen content of cassava, Llanera cultivar. J. Agric. Univ. Puerto Rico59, 115–124.
Garner, W. W., McMurtrey, J. E., Jr., Bowling, J. R., Jr. and Moss, E. G. 1930 Magnesium and calcium requirements of the tobacco crop. J. Agric. Res.40, 145–168.
Goto, Y., Shigeta, K., Ishihara, T. and Yamamoto, M. 1953 Studies on the magnesium deficiency of crops. 2. Chemical relations in cases of wheat and rape. Sci. Rep. Shiga Agric. Coll. (Shiga Pref., Japan)5, 15–18.
Crime, J. P. and Hodgson, J. G. 1969 An investigation of the ecological significance of limechlorosis by means of large scale comparative experiments,In Ecological Aspects of the Mineral Nutrition of Plants, Ed. I. H. Rorison. pp. 67–100, Blackwell Sci. Publ., Oxford and Edinburgh.
Hagen, C. E. and Hopkins, H. T. 1955 Ionic species in orthophosphate absorption by barley roots. Plant Physiol.30, 193–199.
Hendrix, J. E. 1967 The effect of pH on the uptake and accumulation of phosphate and sulphate ions by bean plants. Am. J. Bot.54, 560–564.
Hoagland, D. R. 1917 The effect of hydrogen and hydroxyl ion concentration on the growth of barley seedlings. Soil Sci.3, 547–560.
Howeler, R. H., Cadavid, L. F. and Calvo, F. A. 1977 The interaction of lime with minor elements and phosphorus in cassava production,In Proc. 4th Symp. Int. Soc. Tropical Root Crops. Eds. J. Cock, R. MacIntyre and M. Graham pp. 113–117, IDRC, Ottawa, Canada.
Islam, A. K. M. S., Asher, C. J., Edwards, D. G. and Evenson, J. P. 1978 Germination and early growth of ginger. II. Effects of 2-chloroethylphosphonic acid or elevated temperature pretreatments. Trop. Agric. Trinidad55, 127–134.
Islam, A. K. M. S., Edwards, D. G. and Asher, C. J. 1979 The role of lime in commercial ginger production in Australia. Fifth Symp. Int. Soc. Tropical Root Crops, Manila, Philippines (in press).
Jacobson, L., Moore, D. P. and Hannapel, R. J. 1960 Role of calcium in absorption of monovalent cations. Plant Physiol.35, 352–358.
Jacobson, L., Overstreet, R., King, H. M. and Handley, R. 1950 A study of potassium absorption by barley roots. Plant Physiol.25, 639–647.
Johannesson, K. 1951 Magnesium deficiency in tomato leaves. N. Z. J. Sci. Tech.33A, 52–57.
Johnson, A. D. and Simons, J. G. 1972 Direct reading emission spectroscopic analysis of plant tissue using a briquetting technique. Commun. Soil Sci. Plant Anal.3, 1–9.
Jones, J. B., Jr. 1972 Plant tissue analysis for micronutrients.In Micronutrients in Agriculture. Eds. J. J. Mortvedt et al. pp 319–346. Soil Sci. Soc. Am., Inc., Madison, U.S.A.
Jones, J. P. 1929 Deficiency of magnesium, the cause of chlorosis in corn. J. Agric. Res.39, 873–892.
Kamprath, E. J. 1972 Soil acidity and liming.In Soils of the Humid Tropics, 136–149, Nat. Acad. Sci.—Nat. Res. Council Committee on Tropical Soils, Washington, D.C.
Kashirad, A. and Marschner, H. 1974 Iron nutrition of sunflower plants in mono and mixed culture. Plant and Soil41, 91–101.
Labanauskas, C. K. 1966 Manganese.In Diagnostic Criteria for Plants and Soils. Ed. H. D. Chapman, pp. 264–285, Univ. Calif. Div. Agric. Sci.
Leggett, J. E. and Gilbert, W. A. 1969 Magnesium uptake by soybeans. Plant Physiol.44, 1182–1186.
Lindsay, W. L. and Halvorson, A. D. 1971 Comparison of Fe EDDHA as an iron source for nutrient solutions and for soils. Agron. Abstr. p. 92.
Loneragan, J. F. and Dowling, E. J. 1958 The interaction of calcium and hydrogen ions in the nodulation of subterranean clover. Aust. J. Agric. Res.9, 464–472.
Loneragan, J. F. and Snowball, K. 1969 Calcium requirements of plants. Aust. J. Agric. Res.20, 465–478.
Loneragan, J. F., Snowball, K. and Simmons, W. J. 1968 Response of plants to calcium concentration in solution culture. Aust. J. Agric. Res.19, 845–857.
Maas, E. V. 1969 Calcium uptake by excised maize roots and interactions with alkali cations. Plant Physiol.44, 985–989.
Maas, E. V., Moore, D. P. and Mason, B. J. 1968 Manganese absorption by excised barley roots. Plant Physiol.43, 527–530.
Maas, E. V. and Ogata, G. 1971 Absorption of magnesium and chloride by excised corn roots. Plant Physiol.47, 357–360.
Marschner, H., Handley, R. and Overstreet, R. 1966 Potassium loss and changes in the fine structure of corn root tips induced by H-ion. Plant Physiol.41, 1725–1735.
Moore, D. P., Overstreet, R. and Jacobson, L. 1961 Uptake of magnesium and its interaction with calcium in excised barley roots. Plant Physiol.36, 290–295.
Munns, D. N. 1965 Soil acidity and growth of a legume. II. Reactions of aluminium and phosphate in solution and effects of aluminium, phosphate, calcium, and pH onMedicago sativa L. andTrifolium subterraneum L. in solution culture. Aust. J. Agric. Res.16, 743–755.
Norvell, W. A. 1972 Equilibria of metal chelates in soil solution,In Micronutrients in Agriculture. Eds. J. J. Mortvedt et al. pp. 115–138 Soil Sci. Soc. Am., Inc., Madison, U.S.A.
Peech, M. 1965 Hydrogen-ion-activity,In Methods of Soil Analysis. Ed. C. A. Black Part 2. Chemical and Microbiological Properties, 914–926. Monograph No.9, Am. Soc. Agron., Madison, Wisconsin.
Rao, K. P. and Rains, D. W. 1976 Nitrate absorption by barley. I. Kinetics and energetics. Plant Physiol.57, 55–58.
Rashid, A., Chaudhry, F. M. and Sharif, M. 1976 Micronutrient availability to cereals from calcareous soils. III. Zinc absorption by rice and its inhibition by important ions of submerged soils. Plant and Soil45, 613–623.
Robson, A. D. 1969 Soil factors affecting the distribution of annualMedicago species. J. Aust. Inst. Agric. Sci.35, 154–167.
Robson, A. D. and Loneragan, J. F. 1970 Sensitivity of annualMedicago species to manganese toxicity as affected by calcium and pH Aust. J. Agric. Res.21, 223–232.
Rorison, I. H. 1969 Ecological inferences from laboratory experiments on mineral nutrition,In Ecological Aspects of the Mineral Nutrition of Plants. Ed. I. H. Rorison. pp. 155–176. Blackwell Sci. Publ. Oxford and Edinburgh.
Schofield, R. K. and Taylor, A. W. 1955 The measurement of soil pH. Soil Sci. Soc. Am. Proc.19, 164–167.
Smiley, R. W. and Cook, R. J. 1972 Use and abuse of the soil pH measurement. Phytopath.62, 193–194.
Smith, P. F. 1971 Hydrogen ion toxicity on citrus. J. Am. Soc. Hortic. Sci.96, 462–463.
Spain, J. M., Francis, C. A., Howeler, R. H. and Calvo, F. 1975 Differential species and varietal tolerance to soil acidity in tropical crops and pastures.In Soil Management in Tropical America. Eds. E. Bornemisza and A. Alvarado. pp. 308–329. Soil Sci. Dept., N. Carolina State University, Raleigh, N.C., U.S.A.
Spear, S. N., Asher, C. J. and Edwards, D. G. 1978 Response of cassava, sunflower, and maize to potassium concentration in solution. I. Growth and plant potassium concentration. Field Crops Res.1, 347–361.
Spear, S. N., Edwards, D. G. and Asher, C. J. 1978 Response of cassava, sunflower and maize to potassium concentration in solution. III. Interactions between potassium, calcium and magnesium. Field Crops Res.1, 375–389.
Sprague, H. B. 1964 Ed. Hunger Signs in Crops, 3rd edition. David McKay Co., New York, 461 p.
Sutton, C. D. and Hallsworth, E. G. 1958 Studies on the nutrition of forage legumes. I. The toxicity of low pH and high manganese supply to lucerne, as affected by climatic factors and calcium supply. Plant and Soil9, 305–317.
Taras, M. J., Greenberg, A. E. and Hoak, R. D. 1971 Stannous chloride method,In Standard Methods for the Examination of Waste Water. Eds. M. J. Taras et al. pp. 530–532. APHA, AWWA, WPCF, Washington, U.S.A.
Van den Bergh, J. P. 1969 Distribution of pasture plants in relation to chemical properties of the soil.In Ecological Aspects of the Mineral Nutrition of Plants. Ed. I. H. Rorison. pp. 11–24. Blackwell Sci. Publ., Oxford and Edinburgh.
Wallace, A., El Gazzar, A. and Soufi, S. M. 1968 The role of calcium as a micronutrient and its relationship to other micronutrients. Trans. 9th Int. Congr. Soil Sci., Adelaide, Australia2, 357–366.
Wallace, A., Frolich, E. and Lunt, O. R. 1966 Calcium requirement of higher plants. Nature, London209, 634.
White, R. E. 1969 On the measurement of soil pH. J. Aust. Inst. Agric. Sci.35, 3–14.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Islam, A.K.M.S., Edwards, D.G. & Asher, C.J. pH optima for crop growth. Plant Soil 54, 339–357 (1980). https://doi.org/10.1007/BF02181830
Received:
Revised:
Issue Date:
DOI: https://doi.org/10.1007/BF02181830