Abstract
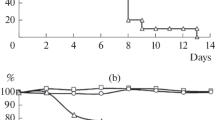
Alice strain live attenuated influenza A (H3N2) virus vaccine was evaluated in prison volunteers. By random double blind allocation, 94 volunteers received Alice strain vaccine (AS) intranasally and 97 received placebo. The vaccine was well tolerated, and there was no serious morbidity. The number, type, duration, and severity of symptoms was not significantly different between the vaccine and placebo groups.
Seventy-five per cent of vaccinees with initial HAI titers less than or equal to 1∶8 had 4 fold or greater titer responses on day 30. Placebo recipients experienced no titer changes. The GMT among vaccinees increased from 23.5 prior to vaccination 59.7 30 days later. Surveillance activities failed to document influenza A (H3N2) infection in the volunteer population during a 6 month follow-up period. Additional studies on the protective effects of the vaccine are required before efficacy can be determined.
Similar content being viewed by others
References
Beare, A. S., Bynoe, M. L.: Attenuation of human influenza A viruses. Brit. med. J.1969 IV, 198–201
Beare, A. S., Hobson, D., Reed, S. E., Tyrrell, D. A. J.: A comparison of live and killed influenza-virus vaccines. Lancet1968 II, 418–420
Freestone, D. S., Hamilton-Smith, S., Schild, G. C., Buckland, R., Chin, S., Tyrrell, D. A. J.: Antibody response and resistance to challenge in volunteers vaccinated with live attenuated, detergent split and oil adjuvant A2/Hong Kong 68 (H3N2) influenza vaccines. J. Hyg. (Lond.)70, 531–534 (1972)
Huygelen, C., Peetermans, J., Vascoboinic, E., Berge, E., Colinet, G.: Live attenuated influenza virus vaccine: “in vitro” and “in vivo” porperties. Symp. Series immunobiol. Standard20, 152–157 (1973)
Kilbourne, E. D., Butler, W. T., Rossen, R. D.: Specific immunity in influenza-summary of influenzy workshop III. J. infect. Dis.127, 220–236 (1973)
Lamy, F., Prinzie, A., Peetermans, J., Vascoboinic, E., Huygelen, C.: Clinical evaluation of live attenuated influenza virus vaccine (“Ann” strain). Symp. Series immunobiol. Standard.20, 282–288 (1973)
Mann, J. J., Waldman, R. H., Togo, Y., Heiner, G. G., Dawkins, A. T., Kasel, J. A.: Antibody response in respiratory secretions of volunteers given live and dead influenza virus. J. Immunol.100, 726–735 (1968)
Miller, L. W., Hume, E. B., O'Brien, F. R., Togo, Y., Hornick, R. B.: Alice strain live attenuated influenza (H3N2) vaccine in an elderly population. Amer. J. Epidemiol.101, 340–346 (1975)
Prevost, J. M., Peetermans, J., Lamy, F., Huygelen, C.: Immune response to vaccination with a live influenza virus (H3N2) vaccine (“Ann” strain). Infect. Immun.8, 420–424 (1973)
Schiff, G. M., Linnemann, C. C., Jr., Shea, L., Lange, B., Rotte, T.: Evaluation of a live, attenuated recombinant influenza vaccine in high school children. Infect. Immun.11, 754–757 (1975)
Smith Kline and French Laboratories, Research and Development Division, Investigational Use Circular; Live attenuated influenza vaccine Alice strain, June 1973
Smith Kline and French Laboratories, Research and Development Division, Investigational Use Circular; Live attenuated influenzy vaccine, September 1974
Wenzel, R. P., Hendley, J., Sande, M. A., Gwaltney, J. M., Jr.: Revised (1972–1973): Bivalent influenza vaccine serum and nasal antibody response to parenteral vaccinations. J. Amer. med. Ass.226, 435–438 (1973)
Author information
Authors and Affiliations
Additional information
This study was aided by a Public Health Service training grant, PHS AH00037-05, and a research contract with Smith, Kline and French Laboratories, Philadelphia, Pennsylvania.
Rights and permissions
About this article
Cite this article
Miller, L.W., Togo, Y. & Hornick, R.B. Clinical and serologic effects of Alice strain live attenuated influenza A (H3N2) virus vaccine in an adult population. Med Microbiol Immunol 162, 15–21 (1975). https://doi.org/10.1007/BF02123573
Received:
Issue Date:
DOI: https://doi.org/10.1007/BF02123573