Abstract
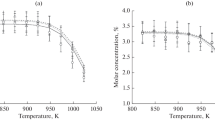
The kinetics of the gas phase pyrolysis of methyl 3-bromopropionate, under maximum catalysis of HBr, were found to be of order 1.0. The reaction appears to undergo a molecular elimination of HBr, which follows first-order kinetics. The products are methyl acrylate and HBr. The pyrolysis, in s static system and seasoned vessel, was examined over the temperature range of 330.5–378.5°C and pressure range of 64–145 Torr. The rate coefficients, under maximum catalysis, are given by the Arrhenius equation log k1(s−1)=(11.19±0.64)−(171.6±7.7) kJ/mol/2.303 RT. The mechanism of the catalyzed pyrolysis of the bromoester appears to proceed through a six-membered cyclic transition state.
Abstract
Кинетика газофазного пиролиза метилового 3-бромпропионата при максимальном катализе HBr-ом имеет порядок, равиый 1,0. При реакции происходит отщепление молекулярного HBr по кинетике первого порядка. Продуктами являются метилакрилат и HBr. Пиролиз в статической системе и высушенном сосуде изучали в интервале температур 330,55–378, 5°C и при давлении 64–145 торр. Константа скорости при максимальном катализе описывается следующим аррениусовским уравнением: log k1 (cek−1)=(11,9±0,64)−(171,6±7,7) кДж/моль/2,303 RT. Механизм
Similar content being viewed by others
References
G. Chuchani, R.M. Domínguez: React. Kinet. Catal. Lett.,27, 185 (1985)
E.S. Swinbourne: “Analysis of Kinetic Data”. p. 50. Nelson, London, 1975.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Chuchani, G., Domínguez, R.M. The maximally catalyzed pyrolysis kinetics of methyl 3-bromopropionate in the gas phase. React Kinet Catal Lett 43, 217–224 (1991). https://doi.org/10.1007/BF02075436
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF02075436