Abstract
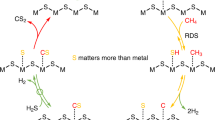
Reaction between methane and sulfur vapor (mainly S2) has been studied at 773–1073 K in the presence of possible catalysts and in the absence of a catalyst. Reaction was carried out in a silica tube reactor at a total pressure of 1 atm with excess methane, with prereaction sulfidation using H2S/H2. Sm2O3 had significant catalytic effect on CH4 conversion, but all other potential catalysts (including Li/MgO) gave CH4 conversion close to that in the absence of catalyst. CS2 was the major product, accompanied by C2 coupling product which in most cases was predominantly C2H4 with some C2H6. In some cases, small amounts of C3, C4 hydrocarbons and CH3SH, (CH3)2S, (CH3)2S2 were also found. Experiments with varying space velocity indicated that C2H6 was the primary coupling product. Possible reaction pathways are discussed in terms of homogeneous CH3·generation, augmented by CH3. generated heterogeneously at the surface of sulfided Sm2O3. Sulfidation of Li/MgO is considered to be the reason for the inactivity of this potential catalyst.
Similar content being viewed by others
References
Kirk-Othmer-Encyclopedia of Chemical Technology: Vol. 22. Wiley, New York 1984.
T. Ito, J. H. Lunsford: Nature (London),314, 721 (1985).
J. R. Anderson: Appl. Catal.,47, 177 (1989).
J. S. Lee, S. T. Oyama: Catal. Rev. Sci. Eng.,30, 249 (1988).
L. A. Bruce, S. Hardin, Manh Hoang, T. W. Turney: J. Materials Chem.,1, 423 (1991).
N. Y. Chen, J. N. Miale, N. Y. Reagan: U.S. Patent, No. 4, 112, 056 (1978).
J. R. Anderson, Y.-F. Chang, R. J. Western: J. Catal.,118, 466 (1989).
L. R. M. Martens, P. J. Grobet, P. A. Jacobs: Nature (London),315, 368 (1985).
I. Kiricsi, I. Hannus, A. Kiss, P. Fejes: Zeolites,2, 247 (1983).
CRC Handbook of Chemistry and Physics, 64th Edn. CRC Press, Cleveland 1983/84.
J.R. West: Ind. Eng. Chem.,42, 713 (1950).
K.K. Kelley: U.S. Bureau of Mines, Bulletin 406 (1937).
The Sulfur Data Book, W.N. Tuller, ed. McGraw-Hill, New York 1954.
B. Meyer: Chem. Rev.,76, 367 (1976).
K. Otsuka, K. Jinno, A. Morikawa: Chem. Lett., 499 (1985).
K. Otsuka, Q. Liu, A. Morikawa: J. Chem. Soc. Chem. Commun., 586 (1986).
K. Otsuka, K. Jinno, A. Morikawa: J. Catal.,100, 353 (1986).
K. Otsuka, K. Jinno: Inorg. Chim. Acta,121, 237 (1986).
TRC Thermodynamic Tables, Texas A and M University, College Station 1986.
S. W. Benson: Thermochemical Kinetics. Wiley, New York 1976.
Synthesis of Lanthanide and Actinide Compounds, G. Meyer, L.R. Morss, eds., Kluwer, Dordrecht 1991.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Anderson, J.R., Chang, Y.F., Pratt, K.C. et al. Reaction of methane and sulfur: Oxidative coupling and carbon disulfide formation. React Kinet Catal Lett 49, 261–269 (1993). https://doi.org/10.1007/BF02067690
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF02067690