Abstract
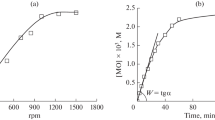
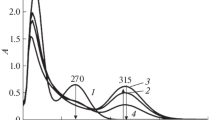
The kinetics of formation of acetic acid, methanol, methyl acetate and hydroperoxide in the autoxidation (463–503 K) and initiated oxidation (413 K) of pentaerythritol tetraacetate has been studied. A scheme for product formation is proposed.
Abstract
Изучены кинетические закономерности образования уксусной кислоты, метанола, метилацетата и гидропероксида при авто (463–503 К) и инициированном (413 К) окислении тетраацетата пентазритрита. Предложена схема образования продуктов.
Similar content being viewed by others
References
V. S. Martemyanov, M. M. Kukovitskii: Neftekhimiya,18, 539 (1978).
E. T. Denisov, N. I. Mitskevich, V. E. Agabekov: Mechanism of Liquid Phase Oxidation of Oxygen-Containing Compounds. Nauka i Technika, Mínsk, 1975.
L. Kerk, M. Feld: J. Am. Chem. Soc.,83, 2998 (1961).
G. G. Agliullina, V. S. Martemyanov, I. A. Ivanova, E. T. Denisov: Izv. Akad. Nauk SSSR, Ser. Khim.,10, 2221 (1977).
E. T. Denisov: Rate Constants of Homolytic Liquid Phase Reactions, p. 31. Nauka, Moskva 1971.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Martemyanov, V.S., Khlebnikov, V.N. & Chushkina, I.A. Kinetics of product formation in the initial phase of oxidative degradation of pentaerythritol tetraacetate. React Kinet Catal Lett 25, 357–362 (1984). https://doi.org/10.1007/BF02064430
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF02064430