Abstract
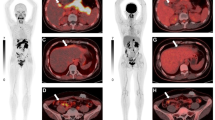
PURPOSE: This study was designed to evaluate a new anticolorectal carcinoma monoclonal antibody (1A3), conjugated with the bifunctional chelating agent N,N′-bis (2-hydroxybenzyl) 1 (4-bromoacetamidobenzyl) 1,2-ethylenediamine-N,N′-diacetic acid and labeled with indium-111, in a Phase I/II study involving 38 patients with localized or advanced colorectal cancer. METHODS: Patients were injected with indium-111-N,N′-bis(2-hydroxybenzyl) 1 (4-bromoacetamidobenzyl) 1,2-ethylenediamine-N, N′-diacetic acid-monoclonal antibody 1A3 (1–50 mg, 1–5 mCi) and imaged at two or three sessions one to five days later. Scintigraphic findings were compared with radiologic, pathologic, surgical, and other clinical findings to assess the accuracy of radioimmunoscintigraphy. RESULTS: At least one known tumor site was clearly defined by planar scintigraphy in 29 (76 percent) patients. Increased radioactivity was seen in 40 of 63 known tumor sites (37/43 abdominal-pelvic, 3/15 hepatic, and 0/5 pulmonary sites) without any apparent dose-related effects. Nineteen previously undetected sites were considered positive by imaging, and, of these, six were biopsy-proven tumor sites, four were probable tumor sites, three were definitely false positive sites, and six were probable false positive sites. Radioimmunoscintigraphy detected proven tumor in 15 of 16 patients with negative or equivocal computed tomography results. Of the 28 patients with rectosigmoid cancer, 25 (89 percent) had positive studies with 34 of 47 tumor sites showing definite uptake on the scintigrams. This included 3 of 9 hepatic metastases. The only adverse reaction occurred in one patient who developed transient hives. Human anti-mouse antibody responses occurred in approximately one-half of the patients injected with doses of 10 or 50 mg. CONCLUSION: This study shows that radioimmunoscintigraphy with this indium-111-labeled monoclonal antibody is safe, it can detect most nonhepatic abdominalpelvic tumors with a positive predictive value of 83 (44/ 53) percent, and it should prove to be useful, particularly in the diagnosis of recurrent rectal carcinoma.
Similar content being viewed by others
References
Baum RP, Hertel A, Schwarz A, Encke A, Hor G.99mTc.labelled anti-CEA monoclonal antibody for tumor immunoscintigraphy: first clinical results. Nucl Med Commun 1989;10:345–50.
Beatty JD, Williams LE, Yamauchi D,et al. Presurgical imaging with indium-labeled anti-carcinoem-bryonic antigen for colon cancer staging. Cancer Res 1990;50:922s-6s.
Bischof-Delaloye A, Delaloye B, Buchegger F,et al. Clinical value of immunoscintigraphy in colorectal carcinoma patients: a prospective study. J Nucl Med 1989;30:1646–56.
Collier BD, Abdel-Nabi H, Doerr RJ,et al. Immunoscintigraphy performed with111In-labeled CYT-103 in the management of colorectal cancer: comparison with CT. Radiology 1992;185:179–86.
Doerr RJ, Abdel-Nabi H, Krag D, Mitchell E. Radiolabeled antibody imaging in the management of colorectal cancer: results of a multicenter clinical study. Ann Surg 1991;214:118–24.
Goldenberg DM, Wlodkowski TJ, Sharkey RM,et al. Colorectal cancer imaging with iodine-123-labeled CEA monoclonal antibody fragments. J Nucl Med 1993;34:61–70.
Granowska M, Mather SJ, Britton KE. Diagnostic evaluation of111In and99mTc radiolabeled monoclonal antibodies in ovarian and colorectal cancer: correlations with surgery. Int J Rad Appl Instrum [B] 1991;18:413–24.
Griffin TH, Brill AB, Stevens S,et al. Initial clinical study of indium-111-labeled clone 110 anticarcinoembryonic antigen antibody in patients with co-lorectal cancer. J Clin Oncol 1991;9:631–40.
Halpern SE, Dillman RO, Amox D,et al. Detection of occult tumor using indium-111 labeled anticarcinoembryonic antigen antibodies. Arch Surg 1992;127:1094–100.
Haseman MK, Brown DW, Keeling CA, Reed NL. Radioimmunodetection of occult carcinoembryonic antigen-producing cancer. J Nucl Med 1992;33:1750–7.
Lamki LM, Patt YZ, Rosenblum MG,et al Metastatic colorectal cancer: radioimmunoscintigraphy with a stabilized111In-labeled F(ab′)2 fragment of an anti-CEA monoclonal antibody. Radiology 1990;174:147–51.
Lind P, Lechner P, Arian-Schad K,et al. Anti-carcinoembryonic antigen immunoscintigraphy (99mTc-monoclonal antibody BW 431/26) and serum CEA levels in patients with suspected primary and recurrent colorectal carcinoma. J Nucl Med 1991;32:1319–25.
Connett JM, Fenwick JR, Timmcke AE, Philpott GW. Characterization of the binding properties of murine monoclonal antibody (MAb) 1A3, a newly described antibody displaying anti-tumor colon cancer selectivity [abstract]. Proc Annu Meet Am Assoc Cancer Res 1987;28:1646.
Connett JM, Inkster MD, Ruiz MB, Philpott GW. Further characterization of murine monoclonal antibody (MAb) 1A3, an antibody displaying anti-human colon cancer selectivity [abstract]. Proc Annu Meet Am Assoc Cancer Res 1988;29:384.
Fenwick JR, Philpott GW, Connett JM. Biodistribution and histological localization of anti-human colon cancer monoclonal antibody (MAb) 1A3: the influence of administered MAb dose on tumor up-take. Int J Cancer 1989;44:1017–37.
Fleshman JW, Connett JM, Neufeld DM, Garvin TJ, Philpott GW. Tumor localization and radioimaging with mixtures of radioiodinated monoclonal antibodies directed to different colon cancer associated antigens. IntJ Rad Appl Instrum [B] 1992;19:659–68.
Hnatowich DJ, Layne WW, Child RL. The preparation and labeling of DTPA-coupled albumin. Int J Rad Appl Instrum [A] 1982;33:327–32.
Krejcarek GE, Tucker KL. Covalent attachment of chelating groups to macromolecules. Biochem Biophys Res Commun 1977;77:581–5.
Paik CH, Ebbert MA, Murphy RR,et al. Factors influencing DTPA conjugation with antibodies by cyclic DTPA anhydride. J Nucl Med 1983;24:1158–63.
Sundberg MW, Meares CF, Goodwin DA, Diamanti CI. Chelating agents for the binding of metal ions to macromolecules. Nature 1974;250:587–8.
Mathias CJ, Sun Y, Connett JM, Philpott GW, Welch MJ, Martell AE. A new bifunctional chelate, Br-Me2HBED: an effective conjugate for radiometals and antibodies. Inorganic Chem 1990;29:1475–80.
Mathias CJ, Sun Y, Welch MJ, Philpott GW, Martell, AE.N,N′-bis-(2-hydroxybenzyl) 1,2-ethylenediamine-N,N′-diacetic acid: a new bifunctional chelate for radiolabeling antibodies. Bioconjug Chem 1990;2:204–11.
Mathias CJ, Sun Y, Welch MJ,et al. Targeting radio-pharmaceuticals: comparative biodistribution studies of gallium and indium complexes of multidentate ligands. IntJ Rad Appl Instrum [B] 1988;15:69–81.
Mathias CJ, Sun Y, Welch MJ, Connett JM, Philpott GW, Martell AE.N,N′-bis-(2-hydroxybenzyl)1-(4-bromoacetamidobenzyl) 1,2-ethylenediamine-N,N′-diacetic acid: a new bifunctional chelate for radio-labeling antibodies. Bioconjug Chem 1990;1:204–11.
Philpott GW, Siegel BA, Schwarz SW, Welch MJ, Connett JM. Initial clinical study of a new indium-labeled anti-colorectal carcinoma monoclonal antibody (MAb 1A3) in patients with advanced colorectal cancer [abstract]. J Nucl Med 1991;32:1054.
Philpott GW, Siegel BA, Schwarz SW, Welch MJ, Connett JM. Improved tumor detection in patients with rectosigmoid cancer using a new anti-colorectal cancer monoclonal antibody (MAb 1A3) [abstract]. Proc Annu Meet Am Soc Clin Oncol 1992;11:164.
Lindmo T, Boven E, Cuttetta F, Fedorko J, Bunn PA Jr. Determination of the immunoreactive fraction of radiolabeled monoclonal antibodies by linear extrapolation to binding at infinite antigen excess. J Immunol Methods 1984;72:77–89.
Hammond ND, Moldofsky PJ, Beardsley MR, Mulhern CB Jr. External imaging techniques for quantitation of distribution of 1-131 F(ab′)2 fragments of monoclonal antibody in humans. Med Phys 1984;11:778–83.
Primus FJ, Kuhns WJ, Goldenberg DM. Immunological heterogeneity of carcinoembryonic antigen: immunohistochemical detection of carcinoembryonic antigen determinants in colonic tumors with monoclonal antibodies. Cancer Res 1983;43:693–701.
Shetye J, Frodin J, Christensson B,et al. Immunohistochemical monitoring of metastatic colorectal carcinoma in patients treated with monoclonal antibodies (MAb 17-1A). Cancer Immunol Immunother 1988;27:154–62.
Rodriguez-Bigas MA, Bakshi S, Stomper P, Blumenson LE, Petrelli NJ.99mTc-Immu-4 monoclonal antibody scan in colorectal cancer: a prospective study. Arch Surg 1992;127:1321–4.
Arnold MW, Schneebaum S, Berens A,et al. Intraoperative detection of colorectal cancer with radioimmunoguided surgery and CC49, a second-generation monoclonal antibody. Ann Surg 1992;216:627–32.
Cohen AM, Martin EW, Lavery I,et al. Radioimmunoguided surgery using iodine-125 B72.3 in patients with colorectal cancer. Arch Surg 1991;126:349–52.
Schlag P, Lehner B, Strauss LG, Georgi P, Herfarth C. Scar or recurrent rectal cancer. Arch Surg 1989;124:197–200.
Abdel-Nabi H, Doerr RJ. Clinical applications of111In-labeled monoclonal antibody imaging in colorectal cancer patients. Semin Nucl Med 1993;23:99–113.
Divgi CR, McDermott K, Johnson DK,et al. Detection of hepatic metastases from colorectal carcinoma using indium-111 (111In) labeled monoclonal antibody (mAb): MSKCC experience with MAb111In-C110. IntJ Rad Appl Instrum [B] 1991;18:705–10.
Author information
Authors and Affiliations
Additional information
This work was performed at the Washington University Medical Center. Supported by National Institutes of Health Grants CA44728 and CA42925 and the Roxanne and Alvin Frank Fund.
Read at the meeting of The American Society of Colon and Rectal Surgeons, Chicago, Illinois, May 2 to 7, 1993.
About this article
Cite this article
Philpott, G.W., Siegel, B.A., Schwarz, S.W. et al. Immunoscintigraphy with a new indium-111-labeled monoclonal antibody (MAb 1A3) in patients with colorectal cancer. Dis Colon Rectum 37, 782–792 (1994). https://doi.org/10.1007/BF02050143
Issue Date:
DOI: https://doi.org/10.1007/BF02050143