Abstract
Prostate-specific membrane antigen (PSMA) is expressed by the majority of clinically significant prostate adenocarcinomas, and patients with target-positive disease can easily be identified by PSMA PET imaging. Promising results with PSMA-targeted radiopharmaceutical therapy have already been obtained in early-phase studies using various combinations of targeting molecules and radiolabels. Definitive evidence of the safety and efficacy of [177Lu]Lu-PSMA-617 in combination with standard-of-care has been demonstrated in patients with metastatic castration-resistant prostate cancer, whose disease had progressed after or during at least one taxane regimen and at least one novel androgen-axis drug. Preliminary data suggest that 177Lu-PSMA-radioligand therapy (RLT) also has high potential in additional clinical situations. Hence, the radiopharmaceuticals [177Lu]Lu-PSMA-617 and [177Lu]Lu-PSMA-I&T are currently being evaluated in ongoing phase 3 trials. The purpose of this guideline is to assist nuclear medicine personnel, to select patients with highest potential to benefit from 177Lu-PSMA-RLT, to perform the procedure in accordance with current best practice, and to prepare for possible side effects and their clinical management. We also provide expert advice, to identify those clinical situations which may justify the off-label use of [177Lu]Lu-PSMA-617 or other emerging ligands on an individual patient basis.
Similar content being viewed by others

Avoid common mistakes on your manuscript.
Preamble
The Society of Nuclear Medicine and Molecular Imaging (SNMMI) is an international scientific and professional organization founded in 1954 to promote the science, technology, and practical application of nuclear medicine. The European Association of Nuclear Medicine (EANM) is a professional non-profit medical association that facilitates communication worldwide between individuals pursuing clinical and research excellence in nuclear medicine. The EANM was founded in 1985. SNMMI and EANM members are physicians, technologists, and scientists specializing in the research and practice of nuclear medicine.
The SNMMI and EANM will periodically define new guidelines for nuclear medicine practice to help advance the science of nuclear medicine and to improve the quality of service to patients throughout the world. Existing practice guidelines will be reviewed for revision or renewal, as appropriate, on their fifth anniversary or sooner, if indicated.
Each practice guideline, representing a policy statement by the SNMMI/EANM, has undergone a thorough consensus process in which it has been subjected to extensive review. The SNMMI and EANM recognize that the safe and effective use of diagnostic nuclear medicine imaging requires specific training, skills, and techniques, as described in each document. Reproduction or modification of the published practice guideline by those entities not providing these services is not authorized.
These guidelines are an educational tool designed to assist practitioners in providing appropriate care for patients. They are not inflexible rules or requirements of practice and are not intended, nor should they be used, to establish a legal standard of care. For these reasons and those set forth below, both the SNMMI and the EANM caution against the use of these guidelines in litigation in which the clinical decisions of a practitioner are called into question.
The ultimate judgment regarding the propriety of any specific procedure or course of action must be made by the physician or medical physicist in light of all the circumstances presented. Thus, there is no implication that an approach differing from the guidelines, standing alone, is below the standard of care. To the contrary, a conscientious practitioner may responsibly adopt a course of action different from that set forth in the guidelines when, in the reasonable judgment of the practitioner, such course of action is indicated by the condition of the patient, limitations of available resources, or advances in knowledge or technology subsequent to publication of the guidelines.
The practice of medicine includes both the art and the science of the prevention, diagnosis, alleviation, and treatment of disease. The variety and complexity of human conditions make it impossible to always reach the most appropriate diagnosis or to predict with certainty a particular response to treatment.
Therefore, it should be recognized that adherence to these guidelines will not ensure an accurate diagnosis or a successful outcome. All that should be expected is that the practitioner will follow a reasonable course of action based on current knowledge, available resources, and the needs of the patient to deliver effective and safe medical care. The sole purpose of these guidelines is to assist practitioners in achieving this objective.
Introduction
Due to its overexpression in most clinically significant prostate adenocarcinomas, the prostate-specific membrane antigen (PSMA) is an important receptor for molecular targeted imaging and therapy. [177Lu]Lu-PSMA-617 proved efficacy in a first phase 3 pivotal trial in patients post taxane and one novel-generation androgen-axis targeting drug by demonstrating superiority compared to the standard-of-care (usually a second novel-generation androgen-axis drug) for the co-primary endpoints of progression-free survival and overall survival and secondary endpoints, e.g., quality of life [1]. Consequently, the label obtained from the U.S. Food and Drug Administration (FDA) in March 2022 and the European Medicines Agency (EMA) in December 2022 only differ in detail and [177Lu]Lu-PSMA-617 will most likely soon become a mainstay of radiopharmaceutical therapy.
Promising anti-tumor-activity has also been reported for the ligand [177Lu]Lu-PSMA-I&T. Academically driven studies and retrospective analyses reported that the pharmacokinetics and dosimetry of [177Lu]Lu-PSMA-I&T and [177Lu]Lu-PSMA-617 are similar [2]. Likewise, protocols of the respective phase 3 trials that evaluate [177Lu]Lu-PSMA-617 (NCT04689828) and [177Lu]Lu-PSMA-I&T (NCT04647526) in the taxane-naïve setting share a common design. Thus, currently, it appears reasonable to consider both compounds broadly equivalent for the purpose of this 177Lu-PSMA-RLT procedure guideline. However, this guideline recognizes that the level of evidence available for [177Lu]Lu-PSMA-I&T is often inferior compared to [177Lu]Lu-PSMA-617, but expects that this will be updated as novel phase 3 evidence becomes available.
In addition to [177Lu]Lu-PSMA-617 and [177Lu]Lu-PSMA-I&T, various PSMA-targeted radiopharmaceuticals based on different ligands or radionuclides are currently under clinical evaluation, e.g., [177Lu]Lu-J591 [3], [177Lu]Lu-DOTA-rosopatamab (NCT04876651), [177Lu]Lu-rhPSMA-10.1 (NCT05413850), [225Ac]Ac-PSMA-617 (NCT04597411), [225Ac]Ac-PSMA-I&T (NCT05219500), [225Ac]Ac-J591 (NCT03276572), [161 Tb]Tb-PSMA-I&T (NCT05521412), [131I]I-1095 (NCT03939689), [227Th]Th-BAY2315497 (NCT03724747), and [67Cu]Cu-SAR-bisPSMA (NCT04868604). It is not our objective to perform a rating of the clinical potential of these approaches since the available clinical evidence does not yet allow final conclusions. Due to their different pharmacokinetics or radiation characteristics, these radiopharmaceuticals are not directly comparable and cannot be incorporated into this generic guideline for 177Lu-PSMA-RLT.
Purpose
The purpose of this guideline is to assist nuclear medicine practitioners in the following:
-
1.
Identifying suitable candidates to receive 177Lu-PSMA-RLT
-
2.
Performing the treatment procedures
-
3.
Understanding the consequences of therapy—i.e., clinical follow-up, management of possible side-effects, response assessment
Definitions
-
1.
177Lu: 177Lutetium is a medium-energy β-emitter. The electron energies (including β-particles and internal conversion electrons) are mean/max 147 keV/497 keV and correspond to ranges of approx. 0.28 mm/1.8 mm in soft tissue. Its physical half-life is 6.65 days. Gamma co-emissions at 208 keV (emission probability 10.4%) and 113 keV (6.2%) enables detection of contaminations and scintigraphy [4, 5].
-
2.
PSMA: It is a transmembrane enzyme with a large extra-cellular domain that is significantly over-expressed on the cell surface of approx. 85% (range 60–100% depending on the definition of PSMA-positive) of prostate adenocarcinomas [6, 7]. With the exception of heterogeneous PSMA expression in neuroendocrine dedifferentiated prostate cancers [8], it positively correlates with grading, i.e., more aggressive tumors tend to have higher expression [9, 10]. Ligand-induced internalization results in partial endosomal trapping of radiolabeled PSMA-ligands [11, 12].
-
3.
RLT: In this guideline, the term radioligand therapy (RLT) is used for the low-molecular-weight (< 10 kDa), Glu-urea-based, PSMA-targeting ligands PSMA-617 and PSMA-I&T, whenever the commentary is generic.
-
4.
CRPC: Briefly, castration-resistant prostate cancer, either metastatic (m-prefix) or non-metastatic (nm-prefix) by imaging, is defined by two consecutive PSA progressions (min. 2 weeks apart) to a 25% increase over nadir or appearance of new lesions on imaging, despite hormonal manipulation leading to testosterone serum levels < 50 ng/dl (< 1.7 nmol/l) [13].
Level of evidence/strength of recommendations
FDA or EMA labels typically rely solely on the protocols and investigator brochures of a few randomized clinical trials (RCTs) [1, 14,15,16,17]. Being based on highly standardized inclusion criteria, RCTs do not necessarily reflect typical real-world patients. Prostate cancer patients are often elderly and affected by several comorbidities that would preclude eligibility for clinical trial involvement. Consequently, the results of RCTs may not be generalizable to their individual clinical situation. To enable recommendations regarding clinical situations where final evidence from prospective RCTs does not exist, a review of the literature was done in December 2021 by searching PubMed.gov for the terms “(PSMA) AND (Lu-177 OR 177Lu OR Lutetium-177) AND (therapy OR theranostics OR dosimetry)” and checking results for appropriateness. A similar search strategy and statistical meta-analysis of the identified studies was performed recently [18, 19]. Clinical experience with PSMA-617 was reported in 53 non-randomized prospective studies and retrospective analyses reflecting > 3600 patients even if some overlap of patient cohorts is considered likely [20,21,22,23,24,25,26,27,28,29,30,31,32,33,34,35,36,37,38,39,40,41,42,43,44,45,46,47,48,49,50,51,52,53,54,55,56,57,58,59,60,61,62,63,64,65,66,67,68,69,70,71,72]; PSMA-I&T was assessed in 11 retrospective analyses reflecting approx. 600 patients [2, 73,74,75,76,77,78,79,80,81,82]. ClinicalTrials.gov identified ongoing phase 3 trials, considered appropriate to amend important novel information within a foreseeable time. A tabular summary of the studies contributing clinical experience regarding safety and anti-tumor-activity, sorted by PSMA-617 vs. PSMA-I&T and retrospective vs. prospective, is provided in the Annex Tables 1, 2 and 3.
If clinical evidence is available from multicenter RCTs with low risk of bias and confounding, a recommendation is classified as “strong” and should apply to most patients in the particular clinical situation. Meta-analysis and systematic reviews of case control or cohort studies or RCTs with moderate risk of bias lead to moderate-strong recommendations. A clinical benefit appears reasonable for subgroups of patients, but individual risk factors must be considered, respectively. This guideline classifies recommendations as “weak” if they are based on case control or cohort studies (higher risk of confounding or bias than RCTs) or studies that only indirectly demonstrate causal relationships. Even case report and expert opinion (based on personal clinical observation) are considered, if they are addressing clinical important questions. However, in situations with only low quality of evidence available, the best action will likely depend on patient’s individual circumstances.
Indications
-
1.
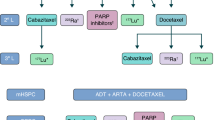
Patients with PSMA-positive mCRPC, who progressed under at least one novel androgen-axis drug (e.g., enzalutamide or abiraterone) and at least one taxane regimen (and are unfit for or refuse a second taxane regimen). Strong recommendation according to highest level of evidence: For this setting, the international phase-3 RCT VISION demonstrated superiority of [177Lu]Lu-PSMA-617 over the best standard-of-care (defined by physician’s choice) regarding safety, efficacy, and quality-of-life [1].
-
2.
Patients with PSMA-positive mCRPC who progressed under at least one novel androgen-axis drug (e.g., enzalutamide or abiraterone) and docetaxel, but would still be possible candidates to receive cabazitaxel: strong recommendation based on a high level of evidence—for this setting, the 11 center phase-2b RCT TheraP demonstrated higher response rates (biochemical, imaging), longer progression free survival at an equal median overall survival, an increased number of long-term responders at 12 months, better patient-reported outcome in multiple domains, and a reduced number of grade 3/4 toxicities of [177Lu]Lu-PSMA-617 compared to cabazitaxel [14].
-
3.
Patients with PSMA-positive but taxane-naïve mCRPC who progressed under at least one novel androgen axis drug (e.g., enzalutamide or abiraterone).
The benefit of 177Lu-PSMA-RLT appears reasonable, and it may be more tolerable than docetaxel for patients with individual contra-indications against docetaxel (moderate strong recommendation for this subgroup of patients). For patients in good general condition who are likely to tolerate docetaxel, the balance between possible benefits and therapy-related risks is still uncertain, and 177Lu-PSMA-RLT cannot be recommended.
One single-center RCT demonstrated equality in PSA response rate, progression-free survival, or grade 3/4 adverse events between [177Lu]Lu-PSMA-617 and docetaxel, but definite conclusions are constrained by low patient numbers (n = 20 in both groups). However, a quality-of-life questionnaire favored [177Lu]Lu-PSMA-617 [15]. Single-arm retrospective analyses reported favorable tolerability and improved response rates of 177Lu-PSMA-RLT in chemotherapy-naïve versus post-taxane patients [20, 73]. However, these single-arm studies did not directly compare 177Lu-PSMA versus docetaxel. Consequently, the level of evidence for this setting is still weak.
Currently, [177Lu]Lu-PSMA-617 and [177Lu]Lu-PSMA-I&T are subject of high quality RCTs evaluating the impact of 177Lu-PSMA-RLT in this stage of disease (Annex Table 4). At present, both radiopharmaceuticals are lacking sufficient clinical evidence and no recommendation for one particular ligand can be given.
-
4.
Currently, various clinical situations are being evaluated in ongoing phase 2/3 RCTs (Annex Table 4) and physicians are encouraged to refer patients to RCTs whenever available. If no option to participate in an RCT exists and alternative treatment options have been exhausted or are contra-indicated, it is reasonable and ethically warranted (Article 37 of Helsinki Declaration) to offer 177Lu-PSMA-RLT on an individual patient basis or in a compassionate care setting; but national regulations have to be considered.
Contra-indications
-
A.
Absolute: As 177Lu-PSMA-RLT is indicated for life-threatening, malignant disease, it is not reasonable to define absolute contra-indications. In general, the chances to improve should outweigh the risks of harming a patient. Therefore, the indication to treat patients with highgrade myelosuppression should be established with caution, and infrastructure to adequately deal with complications should be available.
-
B.
Relative contra-indications: A brief summary of factors that are typically considered as relative contra-indications are provided in Table 1.
Table 1 Relative contra-indications against 177Lu-PSMA-RLT -
C.
Precautions for use:
-
1.
In the VISION trial, combinations with standard-of-care therapy for prostate cancer (analgesics, ADT, NAADs, osteo-protection, and focused radiation therapy) were well-tolerated. No studies regarding the interaction with other medicinal products have been performed.
-
2.
Due to their well-known effects on red-marrow suppression, large-field external beam radiotherapy, chemotherapy, or treatment with radioactive bone-seekers should be discontinued for at least 4 weeks.
-
3.
Clinical experience for patients with moderately reduced kidney function (GFR 30–50 ml/min) is still limited. In patients that started 177Lu-PSMA-RLT with reduced baseline kidney function, renal impairment with probabilities contributable to the standard risk factors for chronic kidney disease alone was reported [21, 74]. However, GFR should carefully be monitored from cycle to cycle and 177Lu-PSMA-RLT should be discontinued if eGFR declines to < 30 ml/min).
-
4.
Patients with reduced kidney function (delayed blood clearance), extensive previous chemotherapy, or history of prolonged hematological toxicity (both indicators of potentially reduced red-marrow reserve) may be more prone to develop higher grade myelotoxicity.
-
5.
Without specific evidence for 177Lu-PSMA-RLT, but according to general knowledge of radiopharmaceuticals: Due to the pro-mutagenic characteristics of any radioactive drug, sufficient contraception is needed during and for 3 months after therapy. Eventually related to the cumulative life dose, the risk to develop irreversible infertility must be considered. The occurrence of therapy-associated myelodysplastic syndrome and secondary leukemia may be increased, particularly in patients with previous extensive exposure to conventional chemotherapy, radio-therapy, or other radiopharmaceutical therapies. These late side effects appear with a delay of several years and are probably not relevant in the setting of mCRPC (even in long-term survivors, they have not been reported), but might be considered if 177Lu-PSMA-RLT is used in earlier stage patients.
Factors associated with survival
Several known prognostic factors (i.e., factors associated with patient outcome, regardless of the specific therapy applied) in mCRPC are also associated with lack of response or short durability of benefit from PSMA-RLT. These prognostic markers can be used by physicians to identify more vulnerable patients. However, due to also being negatively associated with response to several other treatment options, they cannot be used for tailoring therapy [1, 84,85,86].
Prognostic factors associated with poor treatment outcome:
-
1.
Clinical patient/tumor characteristics: Age < 65, ECOG ≥ 2, symptomatic patients, high Gleason score, short response to hormonal intervention (ADT, NAAD).
-
2.
Imaging findings: Presence of visceral metastases, presence of bone-metastases vs. LN-metastases only, extent of bone metastases, total tumor volume, high [18F]FDG-uptake.
-
3.
Lab tests: High PSA, short PSA doubling time, high LDH, high CRP, high ALP, low Hb.
A few predictive factors (i.e., factors associated with clinical outcome of a patient undergoing a specific therapy) have also been identified. These can help stratifying candidate patients to select the most promising subjects for 177Lu-PSMA-RLT and, on the contrary, to identify those for whom 177Lu-PSMA-RLT may not be the best treatment option [22, 23, 85, 87,88,89,90].
Predictive factors associated with sub-standard response to 177Lu-PSMA-RLT:
-
1.
Low tumor uptake of radiolabeled PSMA-ligands; also expressed as low tumor absorbed dose in patient-specific dosimetry, low SUVs in PSMA-PET, low tumor/liver-ratio, or low tumor/parotid-ratio in PSMA-SPECT/scintigraphy.
-
2.
Presence of viable ([18F]FDG-positive) but PSMA-negative tumor lesions.
As PSMA-PET (or PSMA-SPECT) is a strong and relative unique factor for prediction of an individual patient’s response probability to 177Lu-PSMA-RLT, its impact on patient selection is thoroughly discussed in the related chapter of this guideline.
Performing the 177Lu-PSMA-RLT procedure
Facility and personnel prerequisites
Radiopharmaceuticals may be received and administered only by authorized persons in designated facilities that are subject to national regulations. Its radioactive material license must cover appropriate activities of 177Lu. The facility in which the treatment is administered must have appropriate personnel, radiation safety equipment, procedures available for handling and disposal of waste, handling of contamination, and monitoring personnel for accidental spills and controlling contamination spread. Normally, the request for 177Lu-PSMA-RLT will be initiated by an oncologist or urologist, but ideally by an interdisciplinary tumor-board decision. The nuclear medicine specialist is responsible for the 177Lu-PSMA-RLT administration, aftercare, follow-up, and their coordination in close liaison with the referring physicians and other physicians involved in managing the patient. The nuclear medicine specialist is obliged to discuss the technical and clinical aspects of treatment with the patient prior to therapy. A long-term follow-up must be ensured to allow an oncological quality control and detection of possible therapy-related late effects. Involved physicians are encouraged to report any suspected adverse reactions via national reporting systems. Since the treatment is currently standardized and involves the administration of a fixed activity, there is no medical necessity to routinely consult a medical physics expert. However, depending on national legislation and regulations, a medical physics expert should be available for consultation.
In addition to the specific demands of nuclear medicine and radiopharmaceutical therapy, the clinical care for prostate cancer patients generally also involves several other specialists; hence, treatment per multidisciplinary team management (e.g., in a dedicated cancer center) is preferred.
Patient selection
PSMA-targeted therapy will only be effective against tumor lesions with sufficient expression of the target-receptor. The pattern of tumor PSMA expression should be evaluated per molecular imaging (preferred: 68 Ga/18F-PSMA PET; alternatively: 99mTc-PSMA SPECT/scintigraphy) together with a conventional comparator (e.g., CT, MRI, or bone scan) to rule out a relevant fraction of PSMA-negative lymph-node or visceral metastases or active/lytic bone lesions (strong recommendation) [91, 92].
The stringent exclusion of all FDG/PSMA mismatch patients on the TheraP trial reduced patient eligibility, but provided higher PSA-response rates compared to VISION that omitted [18F]FDG-PET/CT evaluation [1, 14]. However, improved overall survival was only proven by VISION, which also recruited patients with small (< 1 cm visceral or < 2.5 cm lymph node) PSMA-negative tumor sites as long as larger lesions demonstrated uptake greater than liver [1, 93]. Thus, simultaneous [18F]FDG-PET appears not mandatory for all patients, but may be helpful in cases with several lesions of uncertain tumor viability or suspicion of PSMA negativity. A conversion from PSMA-positive to PSMA-negative phenotype has repeatedly been reported for liver metastases; hence, especially liver metastases may benefit from a complementary evaluation per [18F]FDG-PET (weak recommendation, based on case reports and expert opinion).
Inter-lesion tumor heterogeneity is common in prostate cancer, and PSMA-immunostaining of a single lesion’s biopsy is not necessarily representative for the majority of other tumor sites [94, 95]. Thus, immunohistochemistry is not considered an appropriate substitute for PSMA-PET/SPECT.
Tumor and normal organ uptake depends on the tracer used for PSMA imaging and SUVs depend on reconstruction parameters and scanner calibration. Regarding the currently most frequently used ligands ([68 Ga]Ga-PSMA-11, [18F]DCFPyL, 18F-PSMA-1007, 18F-rhPSMA-7.3, [99mTc]Tc-PSMA-I&S, [99mTc]Tc-MIP1404), various groups reported that tumor-to-salivary gland or tumor-to-liver ratios can serve as scanner-independent surrogate criteria [96], but in exchange the confounder “reference-organ variability” becomes an issue. Patients with lesions with diameter ≥ 1 cm showing tumor uptake < 0.5-fold of parotid (which approximately equals < 1.0-fold liver-uptake of [68 Ga]Ga-PSMA-11 that has been used in VISION) should be excluded from receiving 177Lu-PSMA-RLT [97]. However, response probability correlates with absorbed dose, which (moderately) correlates with uptake values in PET [87, 90, 98].
PSMA uptake may also be used to determine the optimal sequence of therapies. Preliminary data imply that clinically relevant anti-tumor activity is most likely observed in patients with > onefold parotid uptake in the majority of lesions (approx. SUV > 10), and only such patients should actively be encouraged to receive PSMA-RLT [90]; for patients with low PSMA uptake or negative sites, alternative options should be prioritized.
Recommendations regarding the use of 177Lu-PSMA-RLT beyond its approved indications are challenging. The decision whether a patient is ineligible for a particular alternative treatment is commonly outside the expertise of a nuclear medicine physician alone and should be assessed with the multidisciplinary team. With regard to androgen deprivation therapy (LHRH-analogs/-antagonists and first-generation antiandrogens) and novel androgen-axis drugs (e.g., abiraterone, enzalutamide), the advice of a board-certified urologist, with regard to chemotherapy, and the advice of a board-certified oncologist or a specialized uro-oncologist can be considered sufficient. The decision of an interdisciplinary tumor-board should be preferred. The right of self-determination is a high value in SNMMI and EANM member states, and patients cannot be forced to accept guideline-advised treatment sequences. Nevertheless, patients should be informed that disregarding disease-related guidelines will commonly preclude reimbursement by the public healthcare provider. In any case, it should be documented that the patient has been informed about potential risks and benefits of the alternative treatment options by an expert in the respective field (board-certified uro-/oncologist).
Radiopharmaceuticals
Both [177Lu]Lu-PSMA-617 and [177Lu]Lu-PSMA-I&T belong to the group of PSMA inhibitors that are based on the glutamate-urea-lysine targeting moiety. The precursor PSMA-617 is tagged with a DOTA chelator and has a molar mass of 1042 g/mol (lutetium-labeled: 1216 g/mol). PSMA-I&T is tagged with the DOTAGA chelator, and the precursor molar mass is 1498 g/mol. The sterile therapy solution is typically diluted to ≤ 1000 MBq/ml to reduce the activity concentration in case of extravasation. It is a clear, colorless to slightly yellow solution, free from visible particulates with a radiochemical purity of ≥ 97%.
Currently, only [177Lu]Lu-PSMA-617 is available as an approved drug in the USA, Canada, UK, and EU (177Lu vipivotide tetraxetan, Pluvicto™). The product liability and compliance to Good Manufacturing Practice, according to USP (US Pharmacopeia) or EP (European Pharmacopoeia) standards, is the responsibility of its commercial provider. This formulation should be used for all approved in-label applications of [177Lu]Lu-PSMA-617.
Whenever 177Lu-PSMA-RLT is considered outside the already-approved indications, the aim should be to recruit patients into a formal clinical trial. In this setting, the compliance of the respective radiopharmaceutical to the Investigational Medicinal Product Dossier or the Investigational New Drug application is ensured by the respective sponsor of the trial.
According to article 7 2001/83/EG, in particular situations, drugs can be used without formal approval. National regulations must be considered. Retrospective analyses of “unproven interventions in clinical practice” and some prospectively-performed single-arm studies (Annex, Table 1, 2 and 3) reported safety and efficacy for various formulations of [177Lu]Lu-PSMA-617 and [177Lu]Lu-PSMA-I&T following site-specific, in-house labeling conditions. Advice for production and QC of such formulations is not in the scope of this guideline and should only be done in centers with an adequately qualified radiopharmacy. Otherwise, we recommend off-label use of the approved formulation whenever patients cannot be recruited to clinical trials when it is possible to obtain the drug and full reimbursement.
Process of treatment procedure
-
1.
Patient data, medical history (i.e., previous treatments for prostate cancer, current medication for prostate cancer, and comorbidities), recent staging exams, and current general clinical condition are collected. ADT should be continued during PSMA-RLT; bisphosphonates or denosumab are permitted. In the post-taxane setting, standard-of-care treatment with novel androgen-receptor targeting agents might be given in parallel [1, 99]. Informed consent (written and 24 h in advance of first cycle depending on national legislation) must be obtained.
-
2.
Lab tests not older than 5 days are obtained in advance of each treatment cycle: blood cell count, creatinine, eGFR, AST/GOT, ALT/GPT, total bilirubin, albumin, AP/ALP, LD/LDH, PSA, and CRP.
-
3.
The 177Lu-PSMA radiopharmaceutical solution is stored below 30 °C in its glass vial inside a lead-shielded unit, where the syringe is prepared under aseptic conditions. The activity in the syringe is measured in a dose calibrator (well counter) before and after administration.
-
4.
Sufficient hydration should be ensured by intravenous infusion (e.g., 500–1000 ml of 0.9% saline at a rate of 250 ml/h) starting 30 min in advance and few hours following administration. In compliant patients, oral hydration is also feasible. Patients should be advised to drink a lot of fluids during the next 1–2 weeks.
-
5.
There is no compelling evidence suggesting mandatory co-medication. Prophylactic antiemetic therapy, e.g., ondansetron, is permitted. Corticosteroids before and up to several days after RLT (mandatory in case of cerebral, spinal, or other metastases with risk of painful or obstructive swelling) are allowed per patient’s need and physician’s choice; most experience exists for an average dose of 4 mg dexamethasone given for 5 days [24].
-
6.
A 10-ml saline flush ensures patency of the IV line before administering therapy. 177Lu-PSMA-RLT is slowly administered intravenously over > 30 s and followed by a saline flush with at least twice the volume needed for RLT application.
-
7.
To support kidney clearance, the patient is encouraged to increase fluid intake during the first 3–4 days following treatment. In patients with high tumor burden (increased risk of tumor lysis syndrome), allopurinol can be prescribed during the first week following therapy. Per individual cardiovascular condition, it may be necessary to decrease hydration or to apply diuretics. To reduce bladder dose, patients should void frequently during the first 6–10 h.
-
8.
After administration of the radiopharmaceutical, the patient presents a theoretical risk to other people due to external radiation (gamma co-emissions of 177Lu) or possible exposure to excreted radioactivity. Depending on national legislation, up to 48–72 h isolation on a radioisotope ward may be necessary to reach the local discharge threshold (µSv/h). Where out-patient therapy is allowed, patients should nevertheless be kept in isolation for approx. 2 h to monitor side effects, ensure sufficient hydration, and complete first urination before release. Patients should be instructed how to minimize exposure to others (e.g., avoiding prolonged close contact with pregnant women or infants).
-
9.
At least one planar post-therapeutic emission scan should be obtained > 2 h p.i. to rule out extravasation and confirm physiological tracer biodistribution/excretion [100] (cf. Dosimetry section).
-
10.
The discharge letter should contain relevant patient data, date of therapy, the administered radionuclide (177Lu) and radiopharmaceutical, administered activity, details on necessary aftercare, and next follow-up appointment.
-
11.
In non-compromised patients, the approved treatment regimen for [177Lu]Lu-PSMA-617 is 7.4 GBq (200 mCi) per cycle at 6 w (± 1 w) interval for a maximum of 6 cycles. In clinical practice, safety and anti-tumor activity of [177Lu]Lu-PSMA-617 and [177Lu]Lu-PSMA-I&T have successfully been demonstrated for a range of 6–9.3 GBq per treatment and for treatment intervals of 4–10 weeks [25, 26].
Follow-up
-
1.
Blood cell counts and creatinine should be checked at least 2–3 weeks after each cycle (anticipated nadir) and 4–6 weeks after the last treatment cycle; the consequences of pathological findings are described in the “Management of side effects” section.
-
2.
PSA follow-up should be obtained at each cycle as well as 4–6 weeks after the end of therapy and interpreted in accordance with the PCWG3 criteria [13]. Due to the possibility of PSA flare-up (due to wash-out effects) or delayed response, PSA response becomes a reliable marker 2–3 weeks after the 2nd cycle [27, 28, 101]. Then every increase of > 25% should potentially be considered resistance and trigger imaging-based restaging [29].
-
3.
Scintigraphy (optionally SPECT) of the lutetium gamma co-emissions, 1–2 days after infusion, can serve as an easily available imaging to follow-up response of PSMA-positive lesions. A medium energy collimator is recommended to image the upper 208-keV photo-peak [4, 100].
-
4.
Optimal modality for imaging-based restaging is PSMA-PET/CT. Where PSMA-PET is not available, PSMA-SPECT or scintigraphy is possible. It is mandatory to include a second modality to allow detection of possible PSMA-negative lesions; this may be a fully diagnostic CT as part of an integrated PET/CT exam or a dedicated diagnostic CT; in selected patients, also a bone-scan or [18F]FDG-PET/CT. Frequency and extent of restaging can be adjusted to the reliability of post-treatment scans and PSA response and is usually recommended every 12 weeks and at the end of each series of PSMA-RLT.
-
5.
Assessment of patient-reported well-being (i.e., fatigue, pain, level of activity) is the key parameter for the decision to continue therapy or not.
Management of side effects
Expected side effects include fatigue, acute hematological and chronic renal, or chronic salivary gland toxicity. Grade ≥ 3 side effects occur in < 10% of patients.
-
1.
If acute hematological toxicity (i.e., anemia, leukopenia or neutropenia, thrombocytopenia) of grades 3 or 4 is demonstrated at interim lab test between two cycles: Next cycle should be postponed by 2 weeks and might then be canceled when no recovery is seen. For neutropenia, the use of growth factors (G-CSF) is permitted until toxicity resolves to grade 1, but there should be at least a 2-week interval between G-CSFs and injection of radioactivity. For anemia, transfusion or erythropoietin may be given as clinically indicated.
-
2.
If acute hematological toxicity of ≥ grade 2 is demonstrated at the scheduled treatment date: Treatment activity is reduced by 20% or postponed.
-
3.
Acute loss of eGFR > 40% but still > 30 ml/min: Treatment activity is reduced by 20%.
-
4.
Non-hematological toxicities (e.g., gastrointestinal toxicity, fatigue, electrolyte, or metabolic abnormalities) of grade 3 or 4: Hold 177Lu-PSMA-RLT until recovery to grade 2 or baseline.
-
5.
Skeletal adverse events: Hold 177Lu-PSMA-RLT until complication is adequately treated as deemed appropriate by the treating physician (radiation-oncologist or orthopedic surgeon).
-
6.
Any toxicity that is considered unacceptable by the patient, any life-threatening toxicity that does not resolve within 4 weeks, GFR loss to < 30 ml/min, or unexpected liver toxicity (AST or ALT > fivefold upper-limit of normal): Discontinue 177Lu-PSMA-RLT.
-
7.
Mild cases of xerostomia can be alleviated with coping water or sprays with artificial salivary supplements or xylitol lozenges; moderate strong recommendation.
-
8.
“Unacceptable” xerostomia may eventually be improved by retrograde sialendoscopy and flushing with steroids (weak recommendation based on a single cohort study [102]). Due to its own side effects and contra-indications, the use of parasympathomimetics (e.g., the FDA and EMA approved pilocarpine) must consider patient’s individual situation because benefits and risks could be closely balanced [103].
-
9.
A flare-up of tumor-related pain can occur during the first week following therapy but in responding patients often improves below baseline after the second week. Initial worsening of pain can be caused by radiation-induced edema, best responding to steroids or non-steroidal anti-inflammatory drugs. In severe cases, opioids can temporarily be used. Once interim lab tests demonstrate PSA response, an attempt should be made to reduce analgesics.
Dosimetry
According to the dosimetry sub-study of VISION, the radiation absorbed dose to potentially dose-limiting organs in 29 patients was 2.1 (± 0.47) Gy/GBq for lacrimal and 0.63 (± 0.36) Gy/GBq for salivary glands; kidneys were 0.43 (± 0.16) Gy/GBq and red-marrow 0.035 (± 0.02) Gy/GBq after the first treatment cycle [104]. These doses were extrapolated to all 6 cycles (scaled by a one time-point image for the subsequent cycles) to approximate the cumulative doses for kidneys (19 ± 7.3) Gy and red-marrow (1.5 ± 0.9) Gy with acceptable accuracy [105]. The dosimetry results in this sub-study were consistent with the clinically good safety profile of VISION, with low frequency and severity of radiation-induced adverse events to organs at risk over 6 cycles. Consequently, [177Lu]Lu-PSMA-617 was approved using fixed standard treatment activities, and patient individual dosimetry is not mandatory for in-label use.
Comparable dosimetry estimates have also been obtained in several academically driven dosimetry studies covering various in-house formulations of [177Lu]Lu-PSMA-617 (e.g., with different specific activities GBq/mmol) [22, 23, 31, 36, 61, 70, 87,88,89,90, 106,107,108,109,110,111,112,113,114,115,116,117,118,119,120,121,122] (Annex Table-5). Similar absorbed doses to organs-at-risk have also been approximated when using [177Lu]Lu-PSMA-I&T [75, 81, 98, 123,124,125,126] (Annex Table-5). The technical aspects of the respective studies have recently been summarized in a systematic review [127]. A comparative retrospective analysis, evaluating both radiopharmaceuticals with identical methods, confirmed the similarity of [177Lu]Lu-PSMA-617 and [177Lu]Lu-PSMA-I&T regarding organs at risk [2] but has the limitation of low patient numbers and retrospective matched-pair analysis.
There are different institutional practices regarding post-therapeutic scintigraphy. They are relatively uncommon in some states of the USA. In contrast, according to EC Directive 2013/59/Euratom article 56, at least one post-therapeutic scan can be considered mandatory for therapy verification in most member-states of the EU. However, for standard treatments, a complete dosimetry is optional [128].
As there is significant inter-patient variability, higher treatment activities or more treatment cycles may be possible for several patients under patient individual dosimetry concepts. Recommendations for patient-specific dosimetry that might allow individualized treatment regimens have been provided by the EANM dosimetry committee recently [100]. The clinical status of the patient should be taken into account when considering serial imaging. The ability of the patient to return for additional visits may also be prohibitive if the imaging center is a fair distance away. Novel simplified dosimetry approximation methods, e.g., based on a single posttreatment SPECT/CT scan, have been developed in single-center studies [87, 106]. Whether they can improve clinical outcome by individualized dosimetry-guided treatment regimens still needs confirmation.
Open points/miscellaneous
-
1.
Benefit of additional therapy cycles or later re-treatment in advanced disease (higher cumulative absorbed dose) is still unclear and needs to be studied.
-
2.
The impact of fractionation is still unclear. Different dosing regimens with shorter or longer intervals, more or less sessions and eventually considering individual prognostic factors (e.g., PSA doubling time), need to be studied—especially in the perspective that 177Lu-PSMA-RLT moves into earlier treatment lines.
-
3.
The clinical relevance and potential consequences of the tumor-sink-effect, i.e., (in)variance of normal organ dosimetry at very high tumor load [129, 130], are still unclear and need to be studied.
-
4.
Several drug interventions (e.g., oral glutamate, atropine injections, pre-dosing with unlabeled PSMA ligands) to reduce off-target uptake of PSMA ligands in salivary glands or kidneys have been suggested [103]. Due to the limited evidence available, at this point, no general recommendation can be given. Please pay attention as such interventions may also affect tumor-to-organ ratios that are used for patient selection.
-
5.
It is not clear whether it is needed (and if yes, how) to take prior doses by external-beam radiotherapy (EBRT) or bone-seeking radiopharmaceuticals into account, or conversely, in the case of EBRT after 177Lu-PSMA-RLT (e.g., palliative therapy of painful bone lesions).
-
6.
The prognostic or predictive potential of several molecular biomarkers (e.g., genomic instability, index mutations in DNA or RNA (including splicing variants), tumor stroma or immune related factors) have not sufficiently been evaluated.
-
7.
It remains unclear whether response to previous EBRT has prognostic or predictive impact. However, previous treatment with [223Ra]RaCl2 had no relevant effect on safety and efficacy of a succeeding 177Lu-PSMA-RLT [20].
Liability statement
This guideline summarizes the views of the EANM and SNMMI Committees/Councils for Oncology, Dosimetry, Therapy/Theranostics, PET, Radiation protection, Radiopharmaceutical Sciences and Technologists. It reflects recommendations for which the EANM cannot be held responsible. The recommendations should be taken into context of good practice of nuclear medicine and do not substitute for national and international legal or regulatory provisions.
References
Sartor O, de Bono J, Chi KN, Fizazi K, Herrmann K, Rahbar K, et al. Lutetium-177-PSMA-617 for metastatic castration-resistant prostate cancer. N Engl J Med. 2021;385(12):1091–103. https://doi.org/10.1056/NEJMoa2107322.
Schuchardt C, Zhang J, Kulkarni HR, Chen X, Müller D, Baum RP. Prostate-Specific Membrane Antigen Radioligand Therapy Using 177Lu-PSMA I&T and 177Lu-PSMA-617 in patients with metastatic castration-resistant prostate cancer: comparison of safety, biodistribution, and dosimetry. J Nucl Med. 2022;63(8):1199–207. https://doi.org/10.2967/jnumed.121.262713.
Tagawa ST, Milowsky MI, Morris M, Vallabhajosula S, Christos P, Akhtar NH, et al. Phase II study of Lutetium-177-labeled anti-prostate-specific membrane antigen monoclonal antibody J591 for metastatic castration-resistant prostate cancer. Clin Cancer Res. 2013;19(18):5182–91. https://doi.org/10.1158/1078-0432.CCR-13-0231.
Sjögreen Gleisner K, Chouin N, Gabina PM, Cicone F, Gnesin S, Stokke C, et al. EANM dosimetry committee recommendations for dosimetry of 177Lu-labelled somatostatin-receptor- and PSMA-targeting ligands. Eur J Nucl Med Mol Imaging. 2022;49(6):1778–809. https://doi.org/10.1007/s00259-022-05727-7.
IAEA nuclear data service. Live Chart of Nuclides, nuclear structure and decay data. https://www-nds.iaea.org/relnsd/vcharthtml/VChartHTML.html. Accessed 21 May 2023.
Sweat SD, Pacelli A, Murphy GP, Bostwick DG. Prostate-specific membrane antigen expression is greatest in prostate adenocarcinoma and lymph node metastases. Urology. 1998;52(4):637–40. https://doi.org/10.1016/s0090-4295(98)00278-7.
Mannweiler S, Amersdorfer P, Trajanoski S, Terrett JA, King D, Mehes G. Heterogeneity of prostate-specific membrane antigen (PSMA) expression in prostate carcinoma with distant metastasis. Pathol Oncol Res. 2009;15(2):167–72. https://doi.org/10.1007/s12253-008-9104-2.
Bakht MK, Derecichei I, Li Y, Ferraiuolo RM, Dunning M, Oh SW, et al. Neuroendocrine differentiation of prostate cancer leads to PSMA suppression. Endocr Relat Cancer. 2018;26(2):131–46. https://doi.org/10.1530/ERC-18-0226.
Wright GL Jr, Haley C, Beckett ML, Schellhammer PF. Expression of prostate-specific membrane antigen in normal, benign, and malignant prostate tissues. Urol Oncol. 1995;1(1):18–28. https://doi.org/10.1016/1078-1439(95)00002-y.
Bostwick DG, Pacelli A, Blute M, Roche P, Murphy GP. Prostate specific membrane antigen expression in prostatic intraepithelial neoplasia and adenocarcinoma: a study of 184 cases. Cancer. 1998;82(11):2256–61. https://doi.org/10.1002/(sici)1097-0142(19980601)82:11%3c2256::aid-cncr22%3e3.0.co;2-s .
Liu H, Rajasekaran AK, Moy P, Xia Y, Kim S, Navarro V, et al. Constitutive and antibody-induced internalization of prostate-specific membrane antigen. Cancer Res. 1998;58(18):4055–60.
Rajasekaran SA, Anilkumar G, Oshima E, Bowie JU, Liu H, Heston W, et al. A novel cytoplasmic tail MXXXL motif mediates the internalization of prostate-specific membrane antigen. Mol Biol Cell. 2003;14(12):4835–45. https://doi.org/10.1091/mbc.e02-11-0731.
Scher HI, Morris MJ, Stadler WM, Higano C, Basch E, Fizazi K, et al. Trial design and objectives for castration-resistant prostate cancer: updated recommendations from the prostate cancer clinical trials working group 3. J Clin Oncol. 2016;34(12):1402–18. https://doi.org/10.1200/JCO.2015.64.2702.
Hofman MS, Emmett L, Sandhu S, Iravani A, Joshua AM, Goh JC, et al. [177Lu]Lu-PSMA-617 versus cabazitaxel in patients with metastatic castration-resistant prostate cancer (TheraP): a randomised, open-label, phase 2 trial. Lancet. 2021;397(10276):797–804. https://doi.org/10.1016/S0140-6736(21)00237-3.
Satapathy S, Mittal BR, Sood A, Das CK, Mavuduru RS, Goyal S, et al. 177Lu-PSMA-617 versus docetaxel in chemotherapy-naïve metastatic castration-resistant prostate cancer: a randomized, controlled, phase 2 non-inferiority trial. Eur J Nucl Med Mol Imaging. 2022;49(5):1754–64. https://doi.org/10.1007/s00259-021-05618-3.
Calais J, Gafita A, Eiber M, Armstrong WR, Gartmann J, Thin P, et al. Prospective phase 2 trial of PSMA-targeted molecular RadiothErapy with 177Lu-PSMA-617 for metastatic castration-reSISTant Prostate Cancer (RESIST-PC): efficacy results of the UCLA cohort. J Nucl Med. 2021;62(10):1440–6. https://doi.org/10.2967/jnumed.121.261982.
Calais J, Czernin J, Thin P, Gartmann J, Nguyen K, Armstrong WR, et al. Safety of PSMA-targeted molecular radioligand therapy with 177Lu-PSMA-617: results from the prospective multicenter phase 2 trial RESIST-PC (NCT03042312). J Nucl Med. 2021;62(10):1447–56. https://doi.org/10.2967/jnumed.121.262543.
Sadaghiani MS, Sheikhbahaei S, Werner RA, Pienta KJ, Pomper MG, Gorin MA, et al. 177 Lu-PSMA radioligand therapy effectiveness in metastatic castration-resistant prostate cancer: an updated systematic review and meta-analysis. Prostate. 2022;82(7):826–35. https://doi.org/10.1002/pros.24325.
Sadaghiani MS, Sheikhbahaei S, Werner RA, Pienta KJ, Pomper MG, Solnes LB, et al. A systematic review and meta-analysis of the effectiveness and toxicities of lutetium-177-labeled prostate-specific membrane antigen-targeted radioligand therapy in metastatic castration-resistant prostate cancer. Eur Urol. 2021;80(1):82–94. https://doi.org/10.1016/j.eururo.2021.03.004.
Ahmadzadehfar H, Rahbar K, Baum RP, Seifert R, Kessel K, Bögemann M, et al. Prior therapies as prognostic factors of overall survival in metastatic castration-resistant prostate cancer patients treated with [177Lu]Lu-PSMA-617 A WARMTH multicenter study (the 617. trial). Eur J Nucl Med Mol Imaging. 2021;48(1):113–22. https://doi.org/10.1007/s00259-020-04797-9.
Rosar F, Kochems N, Bartholomä M, Maus S, Stemler T, Linxweiler J, et al. Renal safety of [177Lu]Lu-PSMA-617 radioligand therapy in patients with compromised baseline kidney function. Cancers (Basel). 2021;13(12):3095. https://doi.org/10.3390/cancers13123095.
Maffey-Steffan J, Scarpa L, Svirydenka A, Nilica B, Mair C, Buxbaum S, et al. The 68Ga/177Lu-theragnostic concept in PSMA-targeting of metastatic castration-resistant prostate cancer: impact of post-therapeutic whole-body scintigraphy in the follow-up. Eur J Nucl Med Mol Imaging. 2020;47(3):695–712. https://doi.org/10.1007/s00259-019-04583-2.
Scarpa L, Buxbaum S, Kendler D, Fink K, Bektic J, Gruber L, et al. The 68Ga/177Lu theragnostic concept in PSMA targeting of castration-resistant prostate cancer: correlation of SUVmax values and absorbed dose estimates. Eur J Nucl Med Mol Imaging. 2017;44(5):788–800. https://doi.org/10.1007/s00259-016-3609-9.
Derlin T, Sommerlath Sohns JM, Schmuck S, Henkenberens C, von Klot CAJ, Ross TL, Bengel FM. Influence of short-term dexamethasone on the efficacy of 177Lu-PSMA-617 in patients with metastatic castration-resistant prostate cancer. Prostate. 2020;80(8):619–31. https://doi.org/10.1002/pros.23974.
Rasul S, Hacker M, Kretschmer-Chott E, Leisser A, Grubmüller B, Kramer G, et al. Clinical outcome of standardized 177Lu-PSMA-617 therapy in metastatic prostate cancer patients receiving 7400 MBq every 4 weeks. Eur J Nucl Med Mol Imaging. 2020;47(3):713–20. https://doi.org/10.1007/s00259-019-04584-1.
Rathke H, Giesel FL, Flechsig P, Kopka K, Mier W, Hohenfellner M, et al. Repeated177Lu-Labeled PSMA-617 radioligand therapy using treatment activities of up to 93 GBq. J Nucl Med. 2018;59(3):459–65. https://doi.org/10.2967/jnumed.117.194209.
Rahbar K, Bögeman M, Yordanova A, Eveslage M, Schäfers M, Essler M, Ahmadzadehfar H. Delayed response after repeated 177Lu-PSMA-617 radioligand therapy in patients with metastatic castration resistant prostate cancer. Eur J Nucl Med Mol Imaging. 2018;45(2):243–6. https://doi.org/10.1007/s00259-017-3877-z.
Ahmadzadehfar H, Wegen S, Yordanova A, Fimmers R, Kürpig S, Eppard E, et al. Overall survival and response pattern of castration-resistant metastatic prostate cancer to multiple cycles of radioligand therapy using [177Lu]Lu-PSMA-617. Eur J Nucl Med Mol Imaging. 2017;44(9):1448–54. https://doi.org/10.1007/s00259-017-3716-2.
Kind F, Fassbender TF, Andrieux G, Boerries M, Meyer PT, Ruf J. Early PSA change after [177Lu]PSMA-617 radioligand therapy as a predicator of biochemical response and overall survival. Cancers (Basel). 2021;14(1):149. https://doi.org/10.3390/cancers14010149.
Ahmadzadehfar H, Rahbar K, Kürpig S, Bögemann M, Claesener M, Eppard E, et al. Early side effects and first results of radioligand therapy with (177)Lu-DKFZ-617 PSMA of castrate-resistant metastatic prostate cancer: a two-centre study. EJNMMI Res. 2015;5(1):114. https://doi.org/10.1186/s13550-015-0114-2.
Kratochwil C, Giesel FL, Stefanova M, Benešová M, Bronzel M, Afshar-Oromieh A, et al. PSMA-targeted radionuclide therapy of metastatic castration-resistant prostate cancer with 177Lu-labeled PSMA-617. J Nucl Med. 2016;57(8):1170–6. https://doi.org/10.2967/jnumed.115.171397.
Rahbar K, Schmidt M, Heinzel A, Eppard E, Bode A, Yordanova A, et al. Response and tolerability of a single dose of 177Lu-PSMA-617 in patients with metastatic castration-resistant prostate cancer: a multicenter retrospective analysis. J Nucl Med. 2016;57(9):1334–8. https://doi.org/10.2967/jnumed.116.173757.
Rahbar K, Ahmadzadehfar H, Kratochwil C, Haberkorn U, Schäfers M, Essler M, et al. German multicenter study investigating 177Lu-PSMA-617 radioligand therapy in advanced prostate cancer patients. J Nucl Med. 2017;58(1):85–90. https://doi.org/10.2967/jnumed.116.183194.
Ahmadzadehfar H, Schlolaut S, Fimmers R, Yordanova A, Hirzebruch S, Schlenkhoff C, et al. Predictors of overall survival in metastatic castration-resistant prostate cancer patients receiving [177Lu]Lu-PSMA-617 radioligand therapy. Oncotarget. 2017;8(61):103108–16. https://doi.org/10.18632/oncotarget.21600.
Ahmadzadehfar H, Zimbelmann S, Yordanova A, Fimmers R, Kürpig S, Eppard E, et al. Radioligand therapy of metastatic prostate cancer using 177Lu-PSMA-617 after radiation exposure to 223Ra-dichloride. Oncotarget. 2017;8(33):55567–74. https://doi.org/10.18632/oncotarget.15698.
Fendler WP, Reinhardt S, Ilhan H, Delker A, Böning G, Gildehaus FJ, et al. Preliminary experience with dosimetry, response and patient reported outcome after 177Lu-PSMA-617 therapy for metastatic castration-resistant prostate cancer. Oncotarget. 2017;8(2):3581–90. https://doi.org/10.18632/oncotarget.12240.
Ferdinandus J, Eppard E, Gaertner FC, Kürpig S, Fimmers R, Yordanova A, et al. Predictors of response to radioligand therapy of metastatic castrate-resistant prostate cancer with 177Lu-PSMA-617. J Nucl Med. 2017;58(2):312–9. https://doi.org/10.2967/jnumed.116.178228.
Bräuer A, Grubert LS, Roll W, Schrader AJ, Schäfers M, Bögemann M, Rahbar K. 177Lu-PSMA-617 radioligand therapy and outcome in patients with metastasized castration-resistant prostate cancer. Eur J Nucl Med Mol Imaging. 2017;44(10):1663–70. https://doi.org/10.1007/s00259-017-3751-z.
Rahbar K, Boegemann M, Yordanova A, Eveslage M, Schäfers M, Essler M, Ahmadzadehfar H. PSMA targeted radioligandtherapy in metastatic castration resistant prostate cancer after chemotherapy, abiraterone and/or enzalutamide. A retrospective analysis of overall survival. Eur J Nucl Med Mol Imaging. 2018;45(1):12–9. https://doi.org/10.1007/s00259-017-3848-4.
Khurshid Z, Ahmadzadehfar H, Gaertner FC, Papp L, Zsóter N, Essler M, Bundschuh RA. Role of textural heterogeneity parameters in patient selection for 177Lu-PSMA therapy via response prediction. Oncotarget. 2018;9(70):33312–21. https://doi.org/10.18632/oncotarget.26051.
Hofman MS, Violet J, Hicks RJ, Ferdinandus J, Thang SP, Akhurst T, et al. [177Lu]-PSMA-617 radionuclide treatment in patients with metastatic castration-resistant prostate cancer (LuPSMA trial): a single-centre, single-arm, phase 2 study. Lancet Oncol. 2018;19(6):825–33. https://doi.org/10.1016/S1470-2045(18)30198-0.
Grubmüller B, Senn D, Kramer G, Baltzer P, D’Andrea D, Grubmüller KH, et al. Response assessment using 68Ga-PSMA ligand PET in patients undergoing 177Lu-PSMA radioligand therapy for metastatic castration-resistant prostate cancer. Eur J Nucl Med Mol Imaging. 2019;46(5):1063–72. https://doi.org/10.1007/s00259-018-4236-4.
Yadav MP, Ballal S, Bal C, Sahoo RK, Damle NA, Tripathi M, Seth A. Efficacy and safety of 177Lu-PSMA-617 radioligand therapy in metastatic castration-resistant prostate cancer patients. Clin Nucl Med. 2020;45(1):19–31. https://doi.org/10.1097/RLU.0000000000002833.
Heinzel A, Boghos D, Mottaghy FM, Gaertner F, Essler M, von Mallek D, Ahmadzadehfar H. 68Ga-PSMA PET/CT for monitoring response to 177Lu-PSMA-617 radioligand therapy in patients with metastatic castration-resistant prostate cancer. Eur J Nucl Med Mol Imaging. 2019;46(5):1054–62. https://doi.org/10.1007/s00259-019-4258-6.
van Kalmthout L, Braat A, Lam M, van Leeuwaarde R, Krijger G, Ververs T, et al. First experience with 177Lu-PSMA-617 therapy for advanced prostate cancer in the Netherlands. Clin Nucl Med. 2019;44(6):446–51. https://doi.org/10.1097/RLU.0000000000002561.
Kessel K, Seifert R, Schäfers M, Weckesser M, Schlack K, Boegemann M, Rahbar K. Second line chemotherapy and visceral metastases are associated with poor survival in patients with mCRPC receiving 177Lu-PSMA-617. Theranostics. 2019;9(17):4841–8. https://doi.org/10.7150/thno.35759.
McBean R, O’Kane B, Parsons R, Wong D. Lu177-PSMA therapy for men with advanced prostate cancer: initial 18 months experience at a single Australian tertiary institution. J Med Imaging Radiat Oncol. 2019;63(4):538–45. https://doi.org/10.1111/1754-9485.12891.
Yordanova A, Linden P, Hauser S, Meisenheimer M, Kürpig S, Feldmann G, et al. Outcome and safety of rechallenge [177Lu]Lu-PSMA-617 in patients with metastatic prostate cancer. Eur J Nucl Med Mol Imaging. 2019;46(5):1073–80. https://doi.org/10.1007/s00259-018-4222-x.
Gupta M, Choudhury PS, Rawal S, Karthikeyan G, Talwar V, Dutta KD, Singh A. Safety profile and therapeutic efficacy of one cycle of Lu177-PSMA in end-stage metastatic castration-resistant prostate cancer patients with low performance status. Nucl Med Mol Imaging. 2019;53(6):423–31. https://doi.org/10.1007/s13139-019-00624-8.
Emmett L, Crumbaker M, Ho B, Willowson K, Eu P, Ratnayake L, et al. Results of a prospective phase 2 pilot trial of 177Lu-PSMA-617 therapy for metastatic castration-resistant prostate cancer including imaging predictors of treatment response and patterns of progression. Clin Genitourin Cancer. 2019;17(1):15–22. https://doi.org/10.1016/j.clgc.2018.09.014.
Aghdam RA, Amoui M, Ghodsirad M, Khoshbakht S, Mofid B, Kaghazchi F, et al. Efficacy and safety of 177Lutetium-prostate-specific membrane antigen therapy in metastatic castration-resistant prostate cancer patients: first experience in West Asia - A prospective study. World J Nucl Med. 2019;18(3):258–65. https://doi.org/10.4103/wjnm.WJNM_66_18.
Assadi M, Rezaei S, Jafari E, Rekabpour SJ, Ravanbod MR, Zohrabi F, et al. Potential application of lutetium-177-labeled prostate-specific membrane antigen-617 radioligand therapy for metastatic castration-resistant prostate cancer in a limited resource environment: Initial clinical experience after 2 years. World J Nucl Med. 2020;19(1):15–20. https://doi.org/10.4103/wjnm.WJNM_20_19.
Derlin T, Werner RA, Lafos M, Henkenberens C, von Klot CAJ, Sommerlath Sohns JM, et al. Neuroendocrine differentiation and response to PSMA-targeted radioligand therapy in advanced metastatic castration-resistant prostate cancer: a single-center retrospective study. J Nucl Med. 2020;61(11):1602–6. https://doi.org/10.2967/jnumed.120.241588.
Gadot M, Davidson T, Aharon M, Atenafu EG, Malki A, Levartovsky M, et al. Clinical variables associated with PSA response to lutetium-177-PSMA ([177Lu]-PSMA-617) radionuclide treatment in men with metastatic castration-resistant prostate cancer. Cancers (Basel). 2020;12(5):1078. https://doi.org/10.3390/cancers12051078.
Gafita A, Fendler WP, Hui W, Sandhu S, Weber M, Esfandiari R, et al. Efficacy and safety of 177Lu-labeled prostate-specific membrane antigen radionuclide treatment in patients with diffuse bone marrow involvement: a multicenter retrospective study. Eur Urol. 2020;78(2):148–54. https://doi.org/10.1016/j.eururo.2020.05.004.
Gupta M, Karthikeyan G, Choudhury PS, Sharma A, Singh A, Rawal S. Is 177Lu-PSMA an effective treatment modality for mCRPC patients with bone and visceral metastasis? Hell J Nucl Med. 2020;23(3):312–20. https://doi.org/10.1967/s002449912219.
Leibowitz R, Davidson T, Gadot M, Aharon M, Malki A, Levartovsky M, et al. A retrospective analysis of the safety and activity of lutetium-177-prostate-specific membrane antigen radionuclide treatment in older patients with metastatic castration-resistant prostate cancer. Oncologist. 2020;25(9):787–92. https://doi.org/10.1634/theoncologist.2020-0100.
Marinova M, Alamdar R, Ahmadzadehfar H, Essler M, Attenberger U, Mücke M, Conrad R. Improving quality of life in patients with metastatic prostate cancer following one cycle of 177Lu-PSMA-617 radioligand therapy: a pilot study. Nuklearmedizin. 2020;59(6):409–14. https://doi.org/10.1055/a-1234-5891.
Rathke H, Holland-Letz T, Mier W, Flechsig P, Mavriopoulou E, Röhrich M, et al. Response prediction of 177Lu-PSMA-617 radioligand therapy using prostate-specific antigen, chromogranin A, and lactate dehydrogenase. J Nucl Med. 2020;61(5):689–95. https://doi.org/10.2967/jnumed.119.231431.
Seifert R, Kessel K, Schlack K, Weckesser M, Bögemann M, Rahbar K. Radioligand therapy using [177Lu]Lu-PSMA-617 in mCRPC: a pre-VISION single-center analysis. Eur J Nucl Med Mol Imaging. 2020;47(9):2106–12. https://doi.org/10.1007/s00259-020-04703-3.
Paganelli G, Sarnelli A, Severi S, Sansovini M, Belli ML, Monti M, et al. Dosimetry and safety of 177Lu PSMA-617 along with polyglutamate parotid gland protector: preliminary results in metastatic castration-resistant prostate cancer patients. Eur J Nucl Med Mol Imaging. 2020;47(13):3008–17. https://doi.org/10.1007/s00259-020-04856-1.
Violet J, Sandhu S, Iravani A, Ferdinandus J, Thang SP, Kong G, et al. Long-term follow-up and outcomes of retreatment in an expanded 50-patient single-center phase II prospective trial of 177Lu-PSMA-617 theranostics in metastatic castration-resistant prostate cancer. J Nucl Med. 2020;61(6):857–65. https://doi.org/10.2967/jnumed.119.236414.
Khreish F, Kochems N, Rosar F, Sabet A, Ries M, Maus S, et al. Response and outcome of liver metastases in patients with metastatic castration-resistant prostate cancer (mCRPC) undergoing 177Lu-PSMA-617 radioligand therapy. Eur J Nucl Med Mol Imaging. 2021;48(1):103–12. https://doi.org/10.1007/s00259-020-04828-5.
Michalski K, Klein C, Brueggemann T, Meyer PT, Jilg CA, Ruf J. Assessing response to [177Lu]PSMA radioligand therapy using modified PSMA PET Progression Criteria. J Nucl Med. 2021;62(12):1741–6. https://doi.org/10.2967/jnumed.120.260836.
Prasad V, Huang K, Prasad S, Makowski MR, Czech N, Brenner W. In comparison to PSA, interim Ga-68-PSMA PET/CT response evaluation based on modified RECIST 1.1 after 2nd cycle is better predictor of overall survival of prostate cancer patients treated with 177Lu-PSMA. Front Oncol. 2021;11:578093. https://doi.org/10.3389/fonc.2021.578093.
Rasul S, Hartenbach M, Wollenweber T, Kretschmer-Chott E, Grubmüller B, Kramer G, et al. Prediction of response and survival after standardized treatment with 7400 MBq 177Lu-PSMA-617 every 4 weeks in patients with metastatic castration-resistant prostate cancer. Eur J Nucl Med Mol Imaging. 2021;48(5):1650–7. https://doi.org/10.1007/s00259-020-05082-5.
Rasul S, Wollenweber T, Zisser L, Kretschmer-Chott E, Grubmüller B, Kramer G, et al. Response and toxicity to the second course of 3 cycles of 177Lu-PSMA therapy every 4 weeks in patients with metastatic castration-resistant prostate cancer. Cancers (Basel). 2021;13(10):2489. https://doi.org/10.3390/cancers13102489.
Tatkovic A, McBean R, Wong D. Lu177-PSMA therapy for men with advanced prostate cancer: 18 months survival analysis in a single Australian tertiary institution. J Med Imaging Radiat Oncol. 2021;65(6):740–7. https://doi.org/10.1111/1754-9485.13182.
Widjaja L, Werner RA, Ross TL, Bengel FM, Derlin T. PSMA expression predicts early biochemical response in patients with metastatic castration-resistant prostate cancer under 177Lu-PSMA-617 radioligand therapy. Cancers (Basel). 2021;13(12):2938. https://doi.org/10.3390/cancers13122938.
Privé BM, Peters SMB, Muselaers CHJ, van Oort IM, Janssen MJR, Sedelaar JPM, et al. Lutetium-177-PSMA-617 in low-volume hormone-sensitive metastatic prostate cancer: a prospective pilot study. Clin Cancer Res. 2021;27(13):3595–601. https://doi.org/10.1158/1078-0432.CCR-20-4298.
Yadav MP, Ballal S, Sahoo RK, Tripathi M, Damle NA, Shamim SA, et al. Long-term outcome of 177Lu-PSMA-617 radioligand therapy in heavily pre-treated metastatic castration-resistant prostate cancer patients. PLoS One. 2021;16(5):e0251375. https://doi.org/10.1371/journal.pone.0251375.
Khreish F, Ghazal Z, Marlowe RJ, Rosar F, Sabet A, Maus S, et al. 177 Lu-PSMA-617 radioligand therapy of metastatic castration-resistant prostate cancer: initial 254-patient results from a prospective registry (REALITY Study). Eur J Nucl Med Mol Imaging. 2022;49(3):1075–85. https://doi.org/10.1007/s00259-021-05525-7.
Barber TW, Singh A, Kulkarni HR, Niepsch K, Billah B, Baum RP. Clinical outcomes of 177Lu-PSMA radioligand therapy in earlier and later phases of metastatic castration-resistant prostate cancer grouped by previous taxane chemotherapy. J Nucl Med. 2019;60(7):955–62. https://doi.org/10.2967/jnumed.118.216820.
Gallyamov M, Meyrick D, Barley J, Lenzo N. Renal outcomes of radioligand therapy: experience of 177lutetium-prostate-specific membrane antigen ligand therapy in metastatic castrate-resistant prostate cancer. Clin Kidney J. 2019;13(6):1049–55. https://doi.org/10.1093/ckj/sfz101.
Baum RP, Kulkarni HR, Schuchardt C, Singh A, Wirtz M, Wiessalla S, et al. 177Lu-labeled prostate-specific membrane antigen radioligand therapy of metastatic castration-resistant prostate cancer: safety and efficacy. J Nucl Med. 2016;57(7):1006–13. https://doi.org/10.2967/jnumed.115.168443.
Heck MM, Tauber R, Schwaiger S, Retz M, D’Alessandria C, Maurer T, et al. Treatment outcome, toxicity, and predictive factors for radioligand therapy with 177Lu-PSMA-I&T in metastatic castration-resistant prostate cancer. Eur Urol. 2019;75(6):920–6. https://doi.org/10.1016/j.eururo.2018.11.016.
Kesavan M, Turner JH, Meyrick D, Yeo S, Cardaci G, Lenzo NP. Salvage radiopeptide therapy of advanced castrate-resistant prostate cancer with lutetium-177-labeled prostate-specific membrane antigen: efficacy and safety in routine practice. Cancer Biother Radiopharm. 2018;33(7):274–81. https://doi.org/10.1089/cbr.2017.2403.
Kletting P, Thieme A, Eberhardt N, Rinscheid A, D’Alessandria C, Allmann J, et al. Modeling and predicting tumor response in radioligand therapy. J Nucl Med. 2019;60(1):65–70. https://doi.org/10.2967/jnumed.118.210377.
Acar E, Özdoğan Ö, Aksu A, Derebek E, Bekiş R, Çapa KG. The use of molecular volumetric parameters for the evaluation of Lu-177 PSMA I&T therapy response and survival. Ann Nucl Med. 2019;33(9):681–8. https://doi.org/10.1007/s12149-019-01376-3.
Bülbül O, Ünek İT, Kefi A, Tuna EB, Bekiş R. Factors affecting overall survival and progression-free survival in patients with metastatic castration resistant prostate cancer received 177Lu PSMA I&T therapy. Hell J Nucl Med. 2020;23(3):229–39. https://doi.org/10.1967/s002449912201.
Barna S, Haug AR, Hartenbach M, Rasul S, Grubmüller B, Kramer G, Blaickner M. Dose calculations and dose-effect relationships in 177Lu-PSMA I&T radionuclide therapy for metastatic castration-resistant prostate cancer. Clin Nucl Med. 2020;45(9):661–7. https://doi.org/10.1097/RLU.0000000000003157.
Kesavan M, Meyrick D, Gallyamov M, Turner JH, Yeo S, Cardaci G, Lenzo NP. Efficacy and haematologic toxicity of palliative radioligand therapy of metastatic castrate-resistant prostate cancer with lutetium-177-labeled prostate-specific membrane antigen in heavily pre-treated patients. Diagnostics (Basel). 2021;11(3):515. https://doi.org/10.3390/diagnostics11030515.
Taylor AT, Brandon DC, de Palma D, Blaufox MD, Durand E, Erbas B, et al. SNMMI Procedure Standard/EANM Practice Guideline for diuretic renal scintigraphy in adults with suspected upper urinary tract obstruction 1.0. Semin Nucl Med. 2018;48(4):377–90. https://doi.org/10.1053/j.semnuclmed.2018.02.010.
Suman S, Parghane RV, Joshi A, Prabhash K, Bakshi G, Talole S, et al. Therapeutic efficacy, prognostic variables and clinical outcome of 177Lu-PSMA-617 PRLT in progressive mCRPC following multiple lines of treatment: prognostic implications of high FDG uptake on dual tracer PET-CT vis-à-vis Gleason score in such cohort. Br J Radiol. 2019;92(1104):20190380. https://doi.org/10.1259/bjr.20190380.
Gafita A, Calais J, Grogan TR, Hadaschik B, Wang H, Weber M, et al. Nomograms to predict outcomes after 177Lu-PSMA therapy in men with metastatic castration-resistant prostate cancer: an international, multicentre, retrospective study. Lancet Oncol. 2021;22(8):1115–25. https://doi.org/10.1016/S1470-2045(21)00274-6.
Heidegger I, Kesch C, Kretschmer A, Tsaur I, Ceci F, Valerio M, et al. Biomarkers to personalize treatment with 177Lu-PSMA-617 in men with metastatic castration-resistant prostate cancer - a state of the art review. Ther Adv Med Oncol. 2022;14:17588359221081922. https://doi.org/10.1177/17588359221081922.
Peters SMB, Hofferber R, Privé BM, de Bakker M, Gotthardt M, Janssen M, et al. [68Ga]Ga-PSMA-11 PET imaging as a predictor for absorbed doses in organs at risk and small lesions in [177Lu]Lu-PSMA-617 treatment. Eur J Nucl Med Mol Imaging. 2022;49(4):1101–12. https://doi.org/10.1007/s00259-021-05538-2.
Peters SMB, Privé BM, de Bakker M, de Lange F, Jentzen W, Eek A, et al. Intra-therapeutic dosimetry of [177Lu]Lu-PSMA-617 in low-volume hormone-sensitive metastatic prostate cancer patients and correlation with treatment outcome. Eur J Nucl Med Mol Imaging. 2022;49(2):460–9. https://doi.org/10.1007/s00259-021-05471-4.
Völter F, Mittlmeier L, Gosewisch A, Brosch-Lenz J, Gildehaus FJ, Zacherl MJ, et al. Correlation of an index-lesion-based SPECT dosimetry method with mean tumor dose and clinical outcome after 177Lu-PSMA-617 radioligand therapy. Diagnostics (Basel). 2021;11(3):428. https://doi.org/10.3390/diagnostics11030428.
Violet J, Jackson P, Ferdinandus J, Sandhu S, Akhurst T, Iravani A, et al. Dosimetry of 177Lu-PSMA-617 in metastatic castration-resistant prostate cancer: correlations between pretherapeutic imaging and whole-body tumor dosimetry with treatment outcomes. J Nucl Med. 2019;60(4):517–23. https://doi.org/10.2967/jnumed.118.219352.
Herrmann K, Kraus BJ, Hadaschik B, Kunikowska J, van Poppel H, N’Dow J, et al. Nuclear medicine theranostics comes of age. Lancet Oncol. 2021;22(11):1497–8. https://doi.org/10.1016/S1470-2045(21)00540-4.
Calais J, Czernin J. PSMA expression assessed by PET imaging is a required biomarker for selecting patients for any PSMA-targeted therapy. J Nucl Med. 2021;62(11):1489–91. https://doi.org/10.2967/jnumed.121.263159.
Kuo PH, Benson T, Messmann R, Groaning M. Why we did what we did: PSMA PET/CT selection criteria for the VISION Trial. J Nucl Med. 2022;63(6):816–8. https://doi.org/10.2967/jnumed.121.263638.
Stangl-Kremser J, Rasul S, Tosoian JJ, Salami SS, Zaslavsky A, Udager A, et al. Single-lesion prostate-specific membrane antigen protein expression (PSMA) and response to [177Lu]-PSMA-ligand therapy in patients with castration-resistant prostate cancer. Eur Urol Open Sci. 2021;30:63–6. https://doi.org/10.1016/j.euros.2021.06.007.
Paschalis A, Sheehan B, Riisnaes R, Rodrigues DN, Gurel B, Bertan C, et al. Prostate-specific membrane antigen heterogeneity and DNA repair defects in prostate cancer. Eur Urol. 2019;76(4):469–78. https://doi.org/10.1016/j.eururo.2019.06.030.
Hotta M, Gafita A, Murthy V, Benz MR, Sonni I, Burger I, et al. Predicting the outcome of mCPRC patients after Lu-177 PSMA therapy using semi-quantitative and visual criteria in baseline PSMA PET: an international multicenter retrospective study. J Clin Oncol. 2022;40(6):32–32. https://doi.org/10.1200/JCO.2022.40.6_suppl.032.
Hotta M, Gafita A, Czernin J, Calais J. Outcome of patients with PSMA-PET/CT screen failure by VISION criteria and treated with 177Lu-PSMA therapy: a multicenter retrospective analysis. J Nucl Med. 2022. https://doi.org/10.2967/jnumed.121.263441.
Okamoto S, Thieme A, Allmann J, D’Alessandria C, Maurer T, Retz M, et al. Radiation dosimetry for 177Lu-PSMA I&T in metastatic castration-resistant prostate cancer: absorbed dose in normal organs and tumor lesions. J Nucl Med. 2017;58(3):445–50. https://doi.org/10.2967/jnumed.116.178483.
Suman S, Parghane RV, Joshi A, Prabhash K, Talole S, Basu S. Combined 177 Lu-PSMA-617 PRLT and abiraterone acetate versus 177 Lu-PSMA-617 PRLT monotherapy in metastatic castration-resistant prostate cancer: an observational study comparing the response and durability. Prostate. 2021;81(15):1225–34. https://doi.org/10.1002/pros.24219.
Sjögreen Gleisner K, Chouin N, Gabina PM, Cicone F, Gnesin S, Stokke C, et al. EANM dosimetry committee recommendations for dosimetry of 177Lu-labelled somatostatin-receptor- and PSMA-targeting ligands. Eur J Nucl Med Mol Imaging. 2022;49(6):1778–809. https://doi.org/10.1007/s00259-022-05727-7.
Soydal C, Araz M, Urun Y, Nak D, Ozkan E, Kucuk NO. Prognostic importance of prostatic specific antigen response in patients who received lutetium-177 prostate-specific membrane antigen treatment for castration resistant prostate cancer. Q J Nucl Med Mol Imaging. 2021;65(3):282–6. https://doi.org/10.23736/S1824-4785.19.03165-0.
Rathke H, Kratochwil C, Hohenberger R, Giesel FL, Bruchertseifer F, Flechsig P, et al. Initial clinical experience performing sialendoscopy for salivary gland protection in patients undergoing 225Ac-PSMA-617 RLT. Eur J Nucl Med Mol Imaging. 2019;46(1):139–47. https://doi.org/10.1007/s00259-018-4135-8.
Mahajan S, Grewal RK, Friedman KP, Schöder H, Pandit-Taskar N. Assessment of salivary gland function after 177Lu-PSMA radioligand therapy: current concepts in imaging and management. Transl Oncol. 2022;21:101445. https://doi.org/10.1016/j.tranon.2022.101445.
Full prescribing information for PLUVICTO; FDA 2022. https://www.accessdata.fda.gov/drugsatfda_docs/label/2022/215833s000lbl.pdf?msclkid=d4809f3cab3e11ecbf5d1db14a168354. Accessed 21 May 2023.
Herrmann K, Rahbar K, Eiber M, Krause BJ, Lassmann M, Jentzen W, et al. Dosimetry of 177Lu-PSMA-617 for the treatment of metastatic castration-resistant prostate cancer: results from the VISION trial sub-study. J Clin Oncol. 2022;40(6):97–97. https://doi.org/10.1200/JCO.2022.40.6_suppl.097.
Jackson PA, Hofman MS, Hicks RJ, Scalzo M, Violet J. Radiation dosimetry in 177Lu-PSMA-617 therapy using a single posttreatment SPECT/CT scan: a novel methodology to generate time- and tissue-specific dose factors. J Nucl Med. 2020;61(7):1030–6. https://doi.org/10.2967/jnumed.119.233411.
Kabasakal L, AbuQbeitah M, Aygün A, Yeyin N, Ocak M, Demirci E, Toklu T. Pre-therapeutic dosimetry of normal organs and tissues of (177)Lu-PSMA-617 prostate-specific membrane antigen (PSMA) inhibitor in patients with castration-resistant prostate cancer. Eur J Nucl Med Mol Imaging. 2015;42(13):1976–83. https://doi.org/10.1007/s00259-015-3125-3.
Delker A, Fendler WP, Kratochwil C, Brunegraf A, Gosewisch A, Gildehaus FJ, et al. Dosimetry for (177)Lu-DKFZ-PSMA-617: a new radiopharmaceutical for the treatment of metastatic prostate cancer. Eur J Nucl Med Mol Imaging. 2016;43(1):42–51. https://doi.org/10.1007/s00259-015-3174-7.
Hohberg M, Eschner W, Schmidt M, Dietlein M, Kobe C, Fischer T, et al. Lacrimal glands may represent organs at risk for radionuclide therapy of prostate cancer with [(177)Lu]DKFZ-PSMA-617. Mol Imaging Biol. 2016;18(3):437–45. https://doi.org/10.1007/s11307-016-0942-0.
Yadav MP, Ballal S, Tripathi M, Damle NA, Sahoo RK, Seth A, Bal C. Post-therapeutic dosimetry of 177Lu-DKFZ-PSMA-617 in the treatment of patients with metastatic castration-resistant prostate cancer. Nucl Med Commun. 2017;38(1):91–8. https://doi.org/10.1097/MNM.0000000000000606.
Kabasakal L, Toklu T, Yeyin N, Demirci E, Abuqbeitah M, Ocak M, et al. Lu-177-PSMA-617 prostate-specific membrane antigen inhibitor therapy in patients with castration-resistant prostate cancer: stability, bio-distribution and dosimetry. Mol Imaging Radionucl Ther. 2017;26(2):62–8. https://doi.org/10.4274/mirt.08760.
Khawar A, Eppard E, Sinnes JP, Roesch F, Ahmadzadehfar H, Kürpig S, et al. Prediction of normal organ absorbed doses for [177Lu]Lu-PSMA-617 using [44Sc]Sc-PSMA-617 pharmacokinetics in patients with metastatic castration resistant prostate carcinoma. Clin Nucl Med. 2018;43(7):486–91. https://doi.org/10.1097/RLU.0000000000002102.
Gosewisch A, Delker A, Tattenberg S, Ilhan H, Todica A, Brosch J, et al. Patient-specific image-based bone marrow dosimetry in Lu-177-[DOTA0, Tyr3]-Octreotate and Lu-177-DKFZ-PSMA-617 therapy: investigation of a new hybrid image approach. EJNMMI Res. 2018;8(1):76. https://doi.org/10.1186/s13550-018-0427-z.
Sarnelli A, Belli ML, Di Iorio V, Mezzenga E, Celli M, Severi S, et al. Dosimetry of 177Lu-PSMA-617 after mannitol infusion and glutamate tablet administration: preliminary results of EUDRACT/RSO 2016–002732-32 IRST protocol. Molecules. 2019;24(3):621. https://doi.org/10.3390/molecules24030621.
Wang J, Zang J, Wang H, Liu Q, Li F, Lin Y, et al. Pretherapeutic 68Ga-PSMA-617 PET may indicate the dosimetry of 177Lu-PSMA-617 and 177Lu-EB-PSMA-617 in main organs and tumor lesions. Clin Nucl Med. 2019;44(6):431–8. https://doi.org/10.1097/RLU.0000000000002575.
Gosewisch A, Ilhan H, Tattenberg S, Mairani A, Parodi K, Brosch J, et al. 3D Monte Carlo bone marrow dosimetry for Lu-177-PSMA therapy with guidance of non-invasive 3D localization of active bone marrow via Tc-99m-anti-granulocyte antibody SPECT/CT. EJNMMI Res. 2019;9(1):76. https://doi.org/10.1186/s13550-019-0548-z.
Götz TI, Lang EW, Prante O, Cordes M, Kuwert T, Ritt P, et al. Estimation of [177Lu]PSMA-617 tumor uptake based on voxel-wise 3D Monte Carlo tumor dosimetry in patients with metastasized castration resistant prostate cancer. Nuklearmedizin. 2020;59(5):365–74. https://doi.org/10.1055/a-1204-9932.
Kamaldeep, Wanage G, Sahu SK, Maletha P, Adnan A, Suman S, et al. Examining absorbed doses of indigenously developed 177Lu-PSMA-617 in metastatic castration-resistant prostate cancer patients at baseline and during course of peptide receptor radioligand therapy. Cancer Biother Radiopharm. 2021;36(3):292–304. https://doi.org/10.1089/cbr.2020.3640.
Rosar F, Schön N, Bohnenberger H, Bartholomä M, Stemler T, Maus S, et al. Comparison of different methods for post-therapeutic dosimetry in [177Lu]Lu-PSMA-617 radioligand therapy. EJNMMI Phys. 2021;8(1):40. https://doi.org/10.1186/s40658-021-00385-4.
Kurth J, Heuschkel M, Tonn A, Schildt A, Hakenberg OW, Krause BJ, Schwarzenböck SM. Streamlined schemes for dosimetry of 177Lu-labeled PSMA targeting radioligands in therapy of prostate cancer. Cancers (Basel). 2021;13(15):3884. https://doi.org/10.3390/cancers13153884.
Mahmoudi E, Pirayesh E, Deevband MR, Amoui M, Rad MG, Ghorbani M. Patient-specific dosimetry in radioligand therapy (RLT) for metastatic prostate cancer using 177Lu-DKFZ-PSMA-617. Nucl Med Mol Imaging. 2021;55(5):237–44. https://doi.org/10.1007/s13139-021-00713-7.
Mix M, Renaud T, Kind F, Nemer U, Yousetzadeh-Nowsha E, Moalosi TCG, et al. Kidney doses in 177Lu-based radioligand therapy in prostate cancer: is dose estimation based on reduced dosimetry measurements feasible? J Nucl Med. 2022;63(2):253–8. https://doi.org/10.2967/jnumed.121.262245.
Rinscheid A, Kletting P, Eiber M, Beer AJ, Glatting G. Influence of sampling schedules on [177Lu]Lu-PSMA dosimetry. EJNMMI Phys. 2020;7(1):41. https://doi.org/10.1186/s40658-020-00311-0.
Brosch-Lenz J, Uribe C, Gosewisch A, Kaiser L, Todica A, Ilhan H, et al. Influence of dosimetry method on bone lesion absorbed dose estimates in PSMA therapy: application to mCRPC patients receiving Lu-177-PSMA-I&T. EJNMMI Phys. 2021;8(1):26. https://doi.org/10.1186/s40658-021-00369-4.
Chatachot K, Shiratori S, Chaiwatanarat T, Khamwan K. Patient dosimetry of 177Lu-PSMA I&T in metastatic prostate cancer treatment: the experience in Thailand. Ann Nucl Med. 2021;35(11):1193–202. https://doi.org/10.1007/s12149-021-01659-8.
Feuerecker B, Chantadisai M, Allmann A, Tauber R, Allmann J, Steinhelfer L, et al. Pretherapeutic comparative dosimetry of 177Lu-rhPSMA-7.3 and 177Lu-PSMA I&T in patients with metastatic castration-resistant prostate cancer. J Nucl Med. 2022;63(6):833–9. https://doi.org/10.2967/jnumed.121.262671.
Nautiyal A, Jha AK, Mithun S, Rangarajan V. Dosimetry in Lu-177-PSMA-617 prostate-specific membrane antigen targeted radioligand therapy: a systematic review. Nucl Med Commun. 2022;43(4):369–77. https://doi.org/10.1097/MNM.0000000000001535.
Konijnenberg M, Herrmann K, Kobe C, Verburg F, Hindorf C, Hustinx R, Lassmann M. EANM position paper on article 56 of the Council Directive 2013/59/Euratom (basic safety standards) for nuclear medicine therapy. Eur J Nucl Med Mol Imaging. 2021;48(1):67–72. https://doi.org/10.1007/s00259-020-05038-9.
Filss C, Heinzel A, Miiller B, Vogg ATJ, Langen KJ, Mottaghy FM. Relevant tumor sink effect in prostate cancer patients receiving 177Lu-PSMA-617 radioligand therapy. Nuklearmedizin. 2018;57(1):19–25. https://doi.org/10.3413/Nukmed-0937-17-10.
Tuncel M, Telli T, Tuncalı MÇ, Karabulut E. Predictive factors of tumor sink effect: insights from 177Lu-Prostate-specific membrane antigen therapy. Ann Nucl Med. 2021;35(5):529–39. https://doi.org/10.1007/s12149-021-01593-9.
Acknowledgements
The guidelines were brought to the attention of the relevant EANM Committees and SNMMI councils/centers-of-excellence, Board of Directors and the National Societies of Nuclear Medicine. The comments and suggestions from the involved EANM and the SNMMI Committees/Councils are highly appreciated and have been considered for this Guideline. We also thank for the contributions for the National Delegates and the SNMMI public review.
Funding
Open Access funding enabled and organized by Projekt DEAL.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
C. Kratochwil is a coinventor of PSMA-617 and worked as a scientific consultant for AAA/Novartis, Roche, AdvanCell, Telix. R.J. Hicks is a shareholder of Telix Pharmaceuticals and Founder and Director of PreMIT Pty Ltd. W.J.G. Oyen received speaker fees from Astellas and did advisory boards for AAA/Novartis and Debiopharm. M. Hofman acknowledged philanthropic/government grant support from the Prostate Cancer Foundation (PCF) funded by CANICA Oslo Norway, Peter MacCallum Foundation, Medical Research Future Fund, NHMRC Investigator Grant, Movember, U.S. Department of Defense and the Prostate Cancer Foundation of Australia (PCFA); he received grant support from AAA/Novartis, ANSTO, Bayer, Isotopia, and consulting fees for lectures or advisory boards from Astellas, AstraZeneca, Janssen, Merck/MSD, Mundipharma, and Point Biopharma. K. Kopka is a coinventor of PSMA-617, PSMA-914, and PSMA-1007 and a member of the Scientific Advisory Board of Telix Pharmaceuticals. U. Haberkorn is a coinventor of PSMA-617. W. P. Fendler reports fees from SOFIE Bioscience (research funding), Janssen (consultant, speakers bureau), Calyx (consultant), Bayer (consultant, speakers bureau, research funding), Parexel (image review), Novartis (speakers bureau), and Telix (speakers bureau). M. Eiber reports fees from Blue Earth Diagnostics Ltd. (consultant, research funding), Novartis/AAA (consultant, speaker), Telix (consultant), Bayer (consultant, research funding), RayzeBio (consultant), Point Biopharma (consultant), Eckert-Ziegler (speaker) and Janssen Pharmaceuticals (consultant, speakers bureau), Parexel (image review) and Bioclinica (image review) outside the submitted work and a patent application for rhPSMA. K. Hermann reports personal fees from Bayer, personal fees and other from Sofie Biosciences, personal fees from SIRTEX, non-financial support from ABX, personal fees from Adacap, personal fees from Curium, personal fees from Endocyte, grants and personal fees from Boston Scientific, personal fees from IPSEN, personal fees from Siemens Healthineers, personal fees from GE Healthcare, personal fees from Amgen, personal fees from Novartis, personal fees from ymabs, personal fees from Aktis Oncology, personal fees from Pharma15, personal fees from Theragnostics Ltd., personal fees from Janssen, personal fees from Telix, personal fees from Debiopharm, personal fees from Bain Capital, and personal fees from Eco1R all outside the submitted work. The other authors declared no personal conflict of interest in the field.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
259_2023_6255_MOESM4_ESM.xlsx
Supplementary file4 (XLSX 11.4 KB) Annex-Table 4: Ongoing Clinical Trials with 177Lu-PSMA-617 and 177Lu-PSMA-I&T (registered at https://clinicaltrials.gov/ )
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Kratochwil, C., Fendler, W.P., Eiber, M. et al. Joint EANM/SNMMI procedure guideline for the use of 177Lu-labeled PSMA-targeted radioligand-therapy (177Lu-PSMA-RLT). Eur J Nucl Med Mol Imaging 50, 2830–2845 (2023). https://doi.org/10.1007/s00259-023-06255-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00259-023-06255-8