Abstract
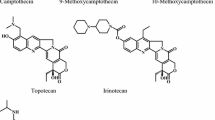
Camptothecin and some of its derivatives, all inhibitors of topoisomerase I, have been found to inhibit growth and induce regression of human colon carcinoma xenografts in nude mice. Some clinical trials of these compounds have been already completed, many more are being held. The mother compound, camptothecin, which is water insoluble, has been administered orally in a Phase I clinical trial. Main toxicity encountered has been diarrhea with minimal leukocytopenia. Camptothecin is now in Phase 2 clinical trials. 9-Aminocamptothecin, a water-insoluble derivative, is now in Phase I trials. Topotecan and iridotecan, two water-soluble derivatives, have undergone Phase I trials, showing mostly intestinal toxicity, followed by bone marrow depression.
Similar content being viewed by others
References
Grage TB, Moss SE. Adjuvant chemotherapy in cancer of the colon and rectum: demonstration of effectiveness of prolonged 5-FU chemotherapy in a prospectively controlled randomized trial. Surg Clin North Am 1981;61:1321–9.
Higgins GA, Lee LE, Dwight RW,et al. The case for adjuvant 5-fluorouracil in colorectal cancer. Cancer Clin Trials 1978;1:35–41.
Higgins GA, Amadeo JH, McElhinney J,et al. Efficacy of prolonged intermittent therapy with combined 5-fluorouracil and me-CCNU following resection for carcinomas of the large bowel. Cancer 1984;53:1–8.
MacDonald JS. Adjuvant therapy of gastrointestinal cancer. In: Salmon SE, ed. Adjuvant therapy of cancer V. New York: Grune & Stratton, 1987:479–96.
Gastrointestinal Tumour Study Group. Adjuvant therapy of colon: results of a prospectively randomized trial. N Engl J Med 1948;310:737–43.
Panettiere FJ, Goodman PJ, Costanzi JJ,et al. Adjuvant therapy in large bowel adenocarcinoma: longterm results of a Southwest Oncology Group study. J Clin Oncol 1988;6:947–54.
Meek AG, Lam WC, Order SE. Carcinoma of the colon: irradiation by delayed split whole-abdominal technique. Radiology 1983;148:845–9.
Brenner JH, Bibi C, Schaitchik S. Adjuvant therapy for Dukes' C adenocarcinoma of the colon. Int J Radiat Oncol Biol Phys 1983;9:1789–92.
Ghossein NA, Samala EC, Alpert S,et al. Elective postoperative radiotherapy after incomplete resection of colorectal cancer. Dis Colon Rectum 1981;24:252–6.
Buroker TR, Moertel CG, Fleming TR,et al. A controlled evaluation of recent approaches to biochemical modulation of enhancement of 5-fluorouracil therapy in colorectal carcinoma. J Clin Oncol 1985;3:1624–31.
Verhaegen H, DeCree J, DeCrock W,et al. Levamisole therapy in patients with colorectal cancer. In: Terry WD, Rosenberg SA, eds. Immunotherapy of human cancer. New York: Excerpta Medica, 1982:225–30.
Chlebowski RT, Nystrom S, Reynolds R,et al. Longterm survival following levamisol or placebo adjuvant treatment of colorectal cancer: a Western oncology group trial. Oncology 1988;45:141–3.
Windle R, Bell PR, Shaw D. Five year results of a randomized trial of adjuvant 5-fluorouracil and levamisole in colorectal cancer. Br J Surg 1987;74:569–72.
Giovanella BC, Wall ME, Wani MC,et al. Highly effective topoisomerase-I targeted chemotherapy of human colon cancer in xenografts. Science 989;246:1046–8.
Wall M, Wani MC, Cook CE,et al. Plant antitumor agents. I. The isolation and structure of camptothecin, a novel alkaloidal leukemia and antitumor fromCamptothecin acuminata. J Am Chem Soc 1966;88:3888–90.
Gottlieb JA, Guarino AM, Call JB,et al. Preliminary pharmacological and clinical evaluation of camptothecin sodium (NSC 100880). Cancer Chemother Rep 1970;54:461–70.
Muggia FM, Creaven PJ, Hansen HH,et al. Phase I clinical trials of weekly and daily treatment with camptothecin (NSC 100880). Correlation with clinical studies. Cancer Chemother Rep 1972;56:515–21.
Gottlieb JA, Luce JK. Treatment of malignant melanoma with camptothecin (NSC 100800). Cancer Chemother Rep 1972;56:103–5.
Moertel CG, Schutt AJ, Reitemeier RJ,et al. Phase II study of camptothecin (NSC 100880) in the treatment of advanced gastrointestinal cancer. Cancer Chemother Rep 1972;56:95–101.
Hsiang Y-H, Herberg R, Hecht S,et al. Camptothecin induced protein-linked DNA breaks via mammalian DNA topoisomerase I. J Biol Chem 1985;260:14873–8.
Hsiang Y-H, Liu LF. Identification and mammalian topoisomerase I as an intracellular target of the anticancer drug camptothecin. Cancer Res 1988;48:1722–6.
Jaxel C, Kohn KW, Wani MC,et al. Structure-activity study of the actions of camptothecin derivatives on mammalian topoisomerase-I. Evidence for a specific receptor site and for a relation to antitumor activity. Cancer Res 1989;49:1465–9.
Hsiang Y-H, Liu LF, Wall ME,et al. DNA topoisomerase I-mediated DNA cleavage and cytotoxicity of camptothecin analogues. Cancer Res 1989;49:4385–9.
Giovanella BC, Stehlin JS, Shepard RC,et al. Correlation between response to chemotherapy of human tumors in patients and nude mice. Cancer 1983;52:1146–52.
Potmesil M, Giovanella BC, Liu LF,et al. Preclinical studies of DNA topoisomerase I-targeted 9-amino and 10,11-methylenedioxy camptothecins. In: Potmesil M, Kohn KW, eds. DNA topoisomerases in cancer. New York: Oxford University Press, 1991.
Giovanella BC, Hinz HR, Kozielski AJ,et al. Complete growth inhibition of human cancer xenografts in nude mice by treatment with 20-(S)-Camptothecin. Cancer Res 1991;51:3052–5.
Giavazzi R, Jessup JM, Campbell De,et al. Experimental nude mouse model in human colorectal cancer liver metastases. J Natl Cancer Inst 1986;77:1303–10.
Kingsbury WD, Boehm JC, Jakay DR,et al. Synthesis of water-soluble (aminoalkyl) camptothecin analogues: inhibition of topoisomerase I and antitumor activity. J Med Chem 1991;34:98–103.
Kunimoto T, Nitta K, Tanaka T,et al. Antitumor activity of 7-ethyl-10[4-(1 piperidino)-1 piperidino] carbonyloxy-camptothecin, a novel water-insoluble derivative of camptothecin against murine tumors. Cancer Res 1987;47:5944–50.
Author information
Authors and Affiliations
About this article
Cite this article
Giovanella, B.C. New perspectives in colon cancer chemotherapy. Dis Colon Rectum 37 (Suppl 2), S96–S99 (1994). https://doi.org/10.1007/BF02048440
Issue Date:
DOI: https://doi.org/10.1007/BF02048440