Abstract
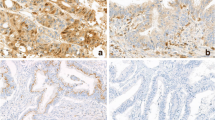
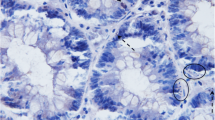
The field change is one hypothesis concerning the development of colorectal carcinoma. Removal of a carcinoma without its entire surrounding altered mucosa may result in the development of a recurrence. S44, a monoclonal antibody directed against statin, a nuclear protein expressed in nonproliferating cells in either a quiescent or senescent state, was used to determine the rate of cell growth in colorectal mucosa at different distances from carcinomas. The specimens of 18 patients undergoing resection of a colorectal carcinoma were immediately opened after operation, and strips of mucosa were taken at distances of 1 cm, 5 cm, and 10 cm from the carcinoma. For each location, 10 longitudinally oriented crypts were evaluated for statin-positive cells identified by the presence of a dark brown peroxidase-conjugated antibody reaction product. The average percentage of statin-positive cells per crypt was significantly lower at a 1-cm distance from the carcinoma compared with the mucosa located 5 and 10 cm from the carcinoma (20.89±4.33 at 1 cm, 32.41±5.27 at 5 cm, and 34.23±6.45 at 10 cm). None of the calculated parameters showed any significant difference between the 5-cm and 10-cm locations. The fact that the proliferation rate of the mucosal cells returns to the normal level at 5 cm from the margin of the carcinoma suggests that cells located within this distance still retain proliferative potential even though they are morphologically indistinguishable from their normal counterparts. We conclude that failure to remove this transitional, potentially proliferative mucosa may result in subsequent development of anastomotic or perianastomotic recurrences.
Similar content being viewed by others
References
Filipe MI. Value of histochemical reactions for mucosubstances in the diagnosis of certain pathological conditions of the colon and rectum. Gut 1969;10:577–86.
Filipe MI. The value of a study of the mucosubstances in rectal biopsies from patients with carcinoma of the rectum and lower sigmoid in the diagnosis of premalignant mucosa. J Clin Pathol 1972;25:123–8.
Rosenberg IL. The etiology of colonic suture line recurrence. Ann R Coll Surg Engl 1979;61:251–7.
Habib NA, Dawson PM, Blount MA, Cox S, Kravitz I, Wood CB. Study of the histochemical changes in mucus from normal and tumor-bearing mucosa in patients with colorectal cancer. Eur J Surg Oncol 1985;11:243–5.
Greaves P, Filipe MI, Branfoot AC. Transitional mucosa and survival in human colorectal cancer. Cancer 1980;46:764–70.
Robey-Cafferty SS, Ro JY, Ordonez G, Cleary KR. Transitional mucosa of colon. A morphological, histochemical and immunohistochemical study. Arch Pathol Lab Med 1990;114:72–5.
Dawson PM, Habib NA, Rees HC, Williamson RC, Wood CB. Influence of sialomucin at the resection margin on local tumor recurrence and survival of patients with colorectal cancer. A multivariate analysis. Br J Surg 1987;74:366–9.
Habib NA, Salem R, Luck RJ, Blount MA, Rifoot MA, Wood CB. A histochemical method that predicts local recurrence after curative resection in carcinoma of the colon and rectum. Surg Gynecol Obstet 1984;159:436–8.
Wood CB, Dawson PM, Habib NA. The sialomucin content of colonic resection margins. Dis Colon Rectum 1985;28:260–1.
Ponz de Leon M, Roncucci L, Di Donato P,et al. Pattern of epithelial cell proliferation in colorectal mucosa of normal subjects and of patients with adenomatous polyp or cancer of the large bowel. Cancer Res 1988;48:4121–6.
Wang E. A 57 kDa molecular weight protein uniquely present in non-proliferating cell and senescent human fibroblasts. J Cell Biol 1985;100:545–51.
Wang E. Statin, a non-proliferation specific protein, is associated with the nuclear envelope and is heterogeneously distributed in cells bearing quiescent state. J Cell Physiol 1989;140:418–26.
Wang E, Krueger JG. Application of a unique monoclonal antibody as a marker for proliferating subpopulations of cells of same tissue. J Histochem Cytochem 1985;33:582–94.
Sester U, Moutsatsos IK, Wang E. A rat liver 57-kDa protein is identified to share antigenic determinants with statin, a marker for non-proliferating cells. Exp Cell Res 1989;182:550–8.
Hsu SM, Raine L, Fanger H. The use of avidin-biotinperoxidase complex (ABC) in immunoperoxidase techniques: a comparison between ABC and unlabelled antibody (PAP) procedures. J Histochem Cytochem 1981;29:577–80.
Bogden A, Gaboreanu M. Techniques of normal and pathological histochemistry. Bucharest: Ceres Publishing, 1976:78–9.
Gill PG, Morris PJ, The survival of patients with colorectal cancer treated in a regional hospital. Br J Surg 1978;65:17–20.
Hickey RL, Romsdah MM, Johnson DE,et al. Recurrent cancer and metastases. World J Surg 1982;6:585–95.
Labow SB, Salvati EP, Rubin RJ. Suture line recurrence in carcinoma of colon and rectum. Dis Colon Rectum 1975;18:123–5.
Long RT, Edwards PH. Implantation metastasis as a cause of local recurrence of colorectal carcinoma. Am J Surg 1989;157:194–201.
Pihl E, Hughes ES, McDermott FT, Price AB. Recurrence of carcinoma of the colon and rectum at the anastomotic suture line. Surg Gynecol Obstet 1981;153:495–6.
Rich T, Gunderson L, Lew R, Galdibini J, Cohen A, Donaldson G. Patterns of recurrence of rectal cancer after potentially curative surgery. Cancer 1983;52:1317–28.
Morson BC, Vaughan EG, Bussey HJ. Pelvic recurrence after excision of the rectum for carcinoma. BMJ 1963;2:13–8.
Olson RM, Perencevich NP, Malcolm AW, Chaffey JT, Wilson RE. Patterns of recurrence following curative resection of adenocarcinoma of the colon and rectum. Cancer 1980;45:2969–74.
Rao AR, Kagan AR, Chan PM, Gilbert HA, Nussbaum H, Hintz BL. Patterns of recurrence following curative resection alone for adenocarcinoma of the rectum and sigmoid colon. Cancer 1981;48:1492–5.
Rosenberg IL. The aetiology of colonic suture line recurrence. Ann R Coll Surg Engl 1979;61:251–7.
Schackert HK, Fidler IJ. Development of an animal model to study the biology of recurrent colorectal cancer originating from mesenteric lymph system metastases. Int J Cancer 1989;44:177–81.
Fermor B, Umpleby HC, Lever JV, Williamson RC. Proliferative and metastatic potential of exfoliated colorectal cancer cells. JNCI 1986;76:347–9.
Symes MO, Fermor B, Umpleby HC, Williamson RC. Cells exfoliated from colorectal cancers can proliferate in immune deprived mice. Br J Cancer 1984;50:423–5.
Umpleby HC, Fermor B, Symes MO, Williamson RC. Viability of exfoliated colorectal carcinoma cells. Br J Surg 1984;71:659–63.
Vink M. Local recurrence of cancer in the large bowel. The role of implantation, metastases and bowel disinfection. Br J Surg 1954;41:431–3.
Lipkin M, Newmark H. Effect of added dietary calcium on colonic epithelial-cell proliferation in subjects at high risk for familial colonic cancer. N Engl J Med 1985;313:1381–4.
Shields HM. Occurrence of an adenocarcinoma at the choledochoenteric anastomosis 14 years after pancreatoduodenectomy for benign disease. Gastroenterology 1977;72:322–4.
Sooriyaarachhi GS, Johnson RO, Carbone PP. Neoplasms of the large bowel following ureterosigmoidostomy. Arch Surg 1977;112:1174–7.
Terpstra OT, Peterson Dahl E, Williamson RC, Ross JS, Malt RA. Colostomy closure promotes cell proliferation and dimethylhydrazine-induced carcinogenesis in rat distal colon. Gastroenterology 1981;81:475–80.
Williamson RC, Bauer FL, Oscarson JE,et al. Promotion of azoxymethane-induced colonic neoplasia by resection of the proximal small bowel. Cancer Res 1978;38:3212–7.
Mori M, Shimono R, Adachi Y,et al. Transitional mucosa in human colorectal lesions. Dis Colon Rectum 1990;33:498–501.
Author information
Authors and Affiliations
Additional information
This study was conducted with support from the Sir Mortimer B. Davis-Jewish General Hospital Foundation and the American Physician Fellowship and with grants to Eugenia Wang from the Medical Research Council of Canada and from the National Institute on Aging of the National Institutes of Health of the U.S.A.
About this article
Cite this article
Kyzer, S., Mitmaker, B., Gordon, P.H. et al. Proliferative activity of colonic mucosa at different distances from primary adenocarcinoma as determined by the presence of statin: A nonproliferation-specific nuclear protein. Dis Colon Rectum 35, 879–883 (1992). https://doi.org/10.1007/BF02047877
Issue Date:
DOI: https://doi.org/10.1007/BF02047877