Abstract
Non-ampullary small bowel adenocarcinoma is a rare neoplasm with an ominous prognosis, whose incidence is higher in some chronic immuno-inflammatory conditions, such as coeliac and Crohn’s disease. Recently, claudin 18.2, a transmembrane protein normally expressed in gastric mucosa, has been recognized as a novel pan-cancer therapeutic target, and several clinical trials with claudin-18-directed drugs have shown promising results on various gastrointestinal malignancies. This is the first study focusing on claudin-18 expression in small bowel adenocarcinomas. The immunohistochemical expression of claudin-18 (clone 43-14A) was assessed in 81 small bowel adenocarcinomas of diverse aetiologies and correlated with several clinico-pathologic features and patient survival. We found that 28% of adenocarcinomas were immunoreactive for claudin-18, with cutoff values of ≥1% at any intensity, while 6% of cancers showed immunoexpression of ≥75% with 2+/3+ score. Moreover, claudin-18 (≥1%) was positively associated with cytokeratin 7 (CK7) and MUC5AC expression, showing CK7+/MUC5AC+ carcinomas the highest rate of positive cases, whereas a negative correlation was found between claudin-18 and CDX2 expression. In addition, some cancer-adjacent dysplastic growths and foci of gastric-type metaplasia in Crohn’s disease-associated cases showed claudin-18 immunoreactivity. Survival analysis showed a non-significant trend towards a worse cancer-specific survival for claudin-18-positive cases. A fraction of small bowel adenocarcinomas, mainly sporadic or Crohn’s disease-associated, and often exhibiting a non-intestinal immunoprofile, expressed claudin-18, suggesting that claudin-18-directed targeted therapy is worth investigating in such cancers.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Non-ampullary small bowel adenocarcinoma (SBA) is a rare epithelial neoplasm representing 30–40% of all cancers [1] of the small intestine and featuring an increasing worldwide incidence [2 3] and a dismal prognosis [4]. Besides de novo sporadic SBAs (Spo-SBAs), hereditary tumour syndromes and chronic immuno-inflammatory conditions represent major risk factors in developing SBA [5 6]. In fact, a substantial proportion of SBAs arises in a background of coeliac disease (CoD) or Crohn’s disease (CrD), each one characterized by quite peculiar clinicopathological features [7 8]. As SBA-related symptoms at presentation are commonly mild and non-specific and an extensive portion of the small intestine cannot be explored by routine endoscopy, diagnosis is often greatly delayed and reached at advanced stages [4 9 10].
Claudin multigene family comprises 27 transmembrane proteins, which takes part in tight junction strands in epithelial cells, playing a pivotal role in tissue homeostasis (i.e. regulating paracellular ion flux and transport and participating in the maintenance of the luminal barrier) and in recruiting signalling proteins [11 12 13]. Particularly, claudin-18 (CLDN18), which can be found in two splicing variants, has a specific topographic expression in healthy tissues, with claudin-18.1 being expressed in the lung and claudin-18.2 in the stomach [14]. In addition, claudin-18.2 was also identified in several epithelial neoplasms, including gastric, pancreatobiliary, oesophageal, colorectal, ovarian and non-small cell lung carcinomas [15 16 17 18]. However, to the best of our knowledge, no study has hitherto investigated CLDN18 expression in SBAs.
Being selectively expressed only in short-lived differentiated gastric cells, other than in some neoplastic tissues, claudin-18.2 was recognized as a safe pan-cancer target [16 19]. Therefore, both monoclonal antibodies (mAb) [20 21 22] and claudin-18.2-specific chimeric antigen receptor engineered T-cells (CAR-T) [23 24] have been recently developed and employed in several clinical trials, showing promising results in the treatment of advanced gastroesophageal and pancreatobiliary tract cancers. Moreover, immunohistochemistry studies on CLDN18 in cancers have demonstrated a certain linkage between its expression and certain histotypes in both gastric [16 25 26] and non-gastric neoplasms [16 27 28], as well as an inverse association with cancer-specific survival and cancer aggressiveness [25 27 29 30]. Interestingly, claudin-18.2 expression was even identified in gastric metaplasia in the background of Barrett’s oesophagus, an early well-recognized event in the development of oesophageal and oesophago-gastric junction adenocarcinoma [31] as well as in pancreatic intraepithelial neoplasia and other precursor lesions of pancreatic ductal adenocarcinoma [32]. Claudin-18.2 ectopic expression therefore appeared to be associated with the activation of specialized genetic programs and with a sort of lineage commitment towards gastric differentiation in several carcinogenic processes [16]. With regard to this, our group has recently described a significant fraction of CrD-SBAs expressing two further non-intestinal/gastric, metaplastic markers, i.e. cytokeratin 7 (CK7) and MUC5AC, both in their invasive components and in the associated non-conventional or atypical lesions, when compared to a control group composed with Spo-SBAs, with resultant, important prognostic implications [33].
The aim of this study was to analyse CLDN18 expression in a rather large series of primary non-ampullary SBAs of different aetiologies and to correlate it with several clinicopathological features and with patient survival.
Materials and methods
Patients with surgically resected, non-hereditary, non-ampullary SBAs collected through the Small Bowel Cancer Italian Consortium, with available, unstained tumour sections were enrolled in this study. The aetiological association with any immuno-inflammatory disorder was ascertained by serology, imaging, clinical and histologic findings. We excluded ampullary adenocarcinomas from the present study, as they have been found to have distinctive features in comparison with non-ampullary duodenal adenocarcinomas [34]. This study was approved by the Ethics Committee of Pavia (protocol number: 20140003980).
Tissue samples were fixed in 4% formaldehyde and processed in paraffin wax. Four-micrometre-thick sections stained with haematoxylin–eosin of all cases were reinvestigated for the following histologic variables: pTNM stage (according to the 8th edition American Joint Committee on Cancer (AJCC) staging system criteria) [35], histologic grade, lymphovascular and perineural invasion. SBAs were histologically subclassified in cohesive (including the glandular-type and the medullary-type) and non-cohesive histotypes (including the poorly cohesive cell-type and the mixed-glandular-poorly cohesive type) [8 36]. The presence of conventional (flat or raised dysplasia) and/or non-conventional precancerous lesions [37] associated with SBAs was recorded.
For immunohistochemistry (IHC), tissue samples were stained using the following antibodies: CLDN18, (clone 43-14A; Roche Ventana), CK7 (clone OV-TL 12/30, Dako), CDX2 (clone DAK-CDX2, Dako), CK20 (clone Ks20.8, Dako), MUC5AC (clone CLH2, Abcam), MUC6 (clone CLH5; Leica Biosystems Newcastle Ltd, Newcastle Upon Tyne, UK), β-catenin (clone 14/Beta-Catenin, BD).
CLDN18 immunoreactivity was evaluated with a quantitative (percentage of stained tumour cells) and semiquantitative method, using a histoscore (H-score), as reported in previous studies [18 26 38]. For H-score assessment, 3+ score was given if tumour cells showed a strong, membranous, circumferential staining; 2+ score if tumour cells had a membranous strong but incomplete staining or a complete, circumferentially faint staining; 1+ score when tumour cells showed a faint, incomplete membranous immunostaining; 0 score when no membranous immunoreactivity was found in neoplastic cells. Thus, tumour cells expressing different intensity scores (3+, 2+, 1+, 0) were evaluated separately in percentage and added up to a total of 100%. H-score was calculated by the formula: H-score = [0 × percentage of negative tumour cells] + [1 × percentage 1+-scored tumour cells] + [2 × percentage of 2+-scored tumour cells] + [3 × percentage of 3+-scored tumour cells]. The maximum value of H-score was 300, for tumours expressing 3+-scored immunoreactivity in 100% tumour cells. SBAs showing ≥ 1% of immunoreactive (at least 1+ intensity) neoplastic cells were considered as positive. Cases with a 2+/3+ score CLDN18 intensity in ≥ 75% of tumour cells, which is the IHC cutoff being used for eligibility in ongoing zolbetuximab clinical trials (NCT03504397; NCT03653507; NCT03816163), were recorded separately. All the other markers were considered as positive when at least 10% of the neoplastic cells were stained, as previously reported [7 8 33]. On the basis of the expression of gastric (MUC6 and MUC5AC) and intestinal (CK20 and CDX2) immunophenotypic markers, cases were subclassified into three immunoprofiles defined as: (i) gastric (i.e. tumours showing immunoreactivity for MUC5AC and/or MUC6, in the absence of both CDX2 and CK20 expression), (ii) intestinal (i.e. neoplasms exhibiting reactivity for CK20 and/or CDX2 and no expression of both MUC5AC and MUC6) and (iii) hybrid (i.e. SBAs showing a concomitant immunoreactivity for at least one intestinal and at least one gastric marker) profiles. Cases with nuclear accumulation of β-catenin in at least 10% of neoplastic cells were recorded as positive. Mismatch repair (MMR) proteins status was assessed using the following antibodies: MLH1 (monoclonal, clone ES05, Dako), MSH2 (monoclonal, clone FE11, Dako), MSH6 (monoclonal, clone EP49, Dako) and PMS2 (monoclonal, clone EP51, Dako); immunostaining of MMR proteins in tumour cells was considered as MMR-proficient (MMRp) if unequivocal nuclear expression of all four MMR proteins was retained, or MMR-deficient (MMRd) if complete loss of nuclear expression of one or more MMR proteins was observed, in the presence of an adequate internal positive control (intra-tumour inflammatory and stromal cells and non-neoplastic cells).
Statistical analysis
The data were described with the mean and standard deviation if continuous and with counts and percentages if categorical; they were compared between groups with the Student t test or the Fisher/χ2 test, respectively. Median follow-up was computed with the reverse Kaplan-Meier method. Follow- up was computed from diagnosis of cancer to death or last available follow-up for censored patients. Hazard ratios (HRs) and 95% confidence intervals (CI) were computed using Cox regression. A two-sided P value<0.05 was considered statistically significant.
Results
Demographic and clinico-pathologic features of SBA cases
We analysed 81 cases of surgical resected, non-ampullary, non-hereditary SBAs, encompassing 35 Spo-SBAs, 31 CrD-associated SBAs (CrD-SBAs) and 15 CoD-associated SBAs (CoD-SBAs), part of which have already been included in previous studies [7 8 33 39 40 41]. Their demographic and clinico-pathologic data are summarized in Table 1.
CLDN18 expression and clinico-pathologic associations in SBAs
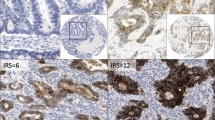
Twenty-three (28%) SBAs showed CLDN18 membranous immunoreactivity in at least 1% of neoplastic cells; the vast majority of these cases had a heterogeneous expression of CLDN18, exhibiting, at different rates, at least two intensity score patterns, with a mean H-score of 68.3 (range 5–270) (Fig. 1).
Clinico-pathologic and immunophenotypic features of the whole cohort and a comparison between CLDN18-positive (≥1% of tumour cells) and CLDN18-negative SBAs (<1%) are summarized in Table 1. A non-significant trend for CLDN18-positive SBAs to occur at older age than CLDN18 negative SBAs was observed (p=0.052). Moreover, CLDN18 expression was less frequent in CoD-SBAs (7%) in comparison with CrD-SBAs (39%) and Spo-SBAs (29%) and less common in jejunal tumours (13%) in comparison with duodenal (30%) or ileal (39%) SBAs, although these differences did not reach statistical significance. A statistically significant association between CLDN18 immunoreactivity (≥1% of tumour cells) and lower pT stage was found (p=0.018).
A strong association was identified between expression of CLDN18 and non-intestinal markers CK7 (p<0.001) and MUC5AC (p<0.001) (Table 1). On the contrary, CLDN18-positive SBAs expressed CDX2 significantly less frequently (39% versus 76%; p=0.002). Stratifying the whole cohort according to the four possible expression patterns for CK7 and MUC5AC, we noted that a great proportion of CLDN18-expressing SBAs (61%) was characterized by a CK7+/MUC5AC+ immunoprofile, while the vast majority of CLDN18-negative SBAs (72%) showed a CK7-/MUC5AC- expression pattern. Interestingly, 14 out of 18 (78%) CK7+/MUC5AC+ SBAs showed immunoreactivity for CLDN18 (Fig. 2). Post hoc comparisons between the four MUC5AC and CK7 expression patterns revealed that CK7+/MUC5AC+ SBAs showed a significantly more common CLDN18 positivity in comparison with CK7-/MUC5AC-SBAs (p<0.001), whereas the other comparisons among CK7/MUC5AC patterns did not reach statistical significance, after Bonferroni correction (Table 1). No significant differences were found between CLDN18-positive and CLDN18-negative cases in terms of MUC6 and CK20 expression.
Stratifying our SBA series according to three possible immunophenotypes (intestinal, gastric and hybrid), the only statistically significant difference in the expression of CLDN18 was found between the intestinal and gastric immunoprofile, with SBAs with a gastric profile showing a more frequent expression of CLDN18 (p<0.001, after Bonferroni correction, Table 1). Post hoc analysis showed no statistically significant differences between gastric versus hybrid and between intestinal versus hybrid profiles. Finally, a negative association between CLDN18 expression and nuclear β-catenin immunoreactivity was identified (p=0.049).
No statistically significant differences between CLDN18-positive and CLDN18-negative SBA cases were found in terms of patient age at diagnosis, patient gender, site, aetiology, AJCC TNM stage, presence of lymph node or distant metastases, lymphovascular or perineural invasion, histologic grade, histotype and MMR status.
Five (6%) SBAs were found to have a moderate-to-strong intensity (i.e. 2+/3+ scores) CLDN18 immunostaining in ≥75% of neoplastic cells (Table 2). Among them, two were located in the ileum, two in the duodenum and one in the jejunum. Of note, all these SBAs arose in male patients, were sporadic, low-grade and with a glandular-type histotype. Most of them (four cases) were MMRp and showed a CK7+/MUC5AC+ immunoprofile. No case with a CLDN18 2+/3+ staining in between 50% and 74% of tumour cells was identified.
Eighteen cases showed 19 dysplastic lesions adjacent to the SBA, encompassing 11 conventional adenomas, 4 flat-type conventional dysplasia and 4 non-conventional dysplastic lesions; all the non-conventional lesions were detected in association with CrD-SBAs, with two of them observed in the same CrD patient. Five (26%) of such dysplastic growths (two CrD-associated flat conventional dysplasias, two CrD-associated non-conventional lesions and one Spo-SBA-associated conventional adenoma) showed CLDN18-positive cells (Fig. 3A). Interestingly, both CLDN18-positive non-conventional dysplastic growths were associated with the same CLDN18-negative CrD-SBA, while the remaining three CLDN18-positive dysplastic lesions were adjacent to CLDN18-immunoreactive SBAs. Moreover, we noticed that foci of foveolar and pyloric metaplastic epithelium and even some scattered normal-appearing crypts in the mucosa in close proximity of CrD-SBAs expressed CLDN18 (Fig. 3B).
Claudin-18 immunohistochemical expression in precursor/preneoplastic lesions next to SBAs. A, A CLDN18-positive conventional adenomatous dysplastic lesion adjacent to a claudin-18-positive sporadic SBA (haematoxylin and eosin; claudin-18 immunostaining in the inlet). B, Claudin-18-positive foveolar metaplasia of the surface epithelium adjacent to an SBA associated with Crohn’s disease (claudin-18 immunohistochemistry)
Survival analysis
Seventy-eight patients were followed up for a median of 41 months, while no follow-up data was available for three cases. Survival analysis showed a trend towards a worse cancer-specific survival for the CLDN18-positive cases in comparison with CLDN18-negative cases, although it did not reach statistical significance (HR: 2.1, 95% CI: 0.98–5.04; p value: 0.078).
Discussion
To date, this is the first study to describe the expression of CLDN18 in a fairly large series of non-ampullary SBAs, including those associated with predisposing inflammatory conditions, and to evaluate its association with several clinico-pathologic features. We found that 28% and 6% of SBAs resulted positive for CLDN18 expression with cutoff values of ≥1% of neoplastic cells at any intensity and ≥75% with 2+/3+ score, respectively. Interestingly, the vast majority of CLDN18-immunoreactive cases were Spo-SBAs or CrD-SBAs, while the prevalence of CLDN18-positivity in CoD-SBAs was low (7%).
Lower rates of CLDN18 positivity were recorded in comparison with those reported in gastroesophageal and bilio-pancreatic cancers [18 20 21 22 25 26 28 42]. Conversely, SBAs expressed CLDN18 more frequently than colorectal carcinomas (CRCs) [27], but, of note, expression rates became similar when CRC cases were enriched, as our series was, with inflammatory bowel disease (IBD)-associated tumours. Moreover, as previously described by Iwaya et al. in the colorectal counterpart [17], we detected CLDN18 expression in tumour-adjacent metaplastic mucosa and, focally, in some scattered, apparently normal crypts of CrD patients only. In our cohort, mostly in CrD-SBAs, CLDN18 positivity was also observed in some cancer-adjacent dysplastic lesions, and in most cases there was concordance in CLDN18 expression between the dysplastic growth and the invasive neoplasm. Our findings are in keeping with prior observations of CLDN18 expression in other gastrointestinal metaplastic and preinvasive lesions (i.e. Barrett’s oesophagus and pancreatic intraepithelial neoplasia) [31 32]. Furthermore, we found a statistically significant association of CLDN18 with both the expression of the non-intestinal markers MUC5AC and CK7 separately evaluated and the MUC5AC+/CK7+ immunophenotype. Interestingly, a correlation of CLDN18 and MUC5AC was previously described in IBD-associated CRCs [17 27]. The increased expression of other metaplastic markers (e.g. MUC5AC, CK7 and CLDN18) in both CrD-SBAs and associated non-neoplastic mucosa [33] may suggest an early lineage commitment towards gastric differentiation in inflamed CrD mucosa, possibly promoting dysplastic and, subsequently, neoplastic evolution. Indeed, while in the stomach the loss of CLDN18 protein was reported to promote inflammation and tumorigenesis, as described in knockout models [43], de novo expression of CLDN18 in small bowel and colon-rectum of IBD patients seemed to be part of the inflammation-metaplasia-dysplasia-cancer process [44, 45]. In this regard, up to now, very scarce knowledge is available, and further investigations are required.
As expected, in line with another study on gastric neoplasms [27], CLDN18 was negatively associated with CDX2 expression in SBAs also. This finding suggests, in the light of the low expression of CLDN18 expression in CDX2-positive gastric cancers [46], a strong influence of the activation of highly specific intestinal transcription factor CDX2 in the differentiation of neoplastic cells towards an intestinal phenotype. The higher expression of intestinal differentiation markers described in CoD-SBAs in comparison with CrD-SBAs [8] may contribute to explain the low prevalence of CLDN18 positivity in CoD-SBAs.
Furthermore, we noted a negative correlation between CLDN18 and nuclear translocation of β-catenin. Nevertheless, CLDN18 has been described to have a two-faced behaviour towards β-catenin, both inhibiting and enhancing its expression [13]; thus, further molecular studies are needed to shed light on this correlation. Finally, we showed a non-significant trend towards a worse cancer-specific survival in CLDN18-positive SBAs, as reported in CRC [27], even though very discordant data are reported in the Literature for other gastrointestinal cancers [25 26 28 37 42 47 48].
Our study has some limitations, including its retrospective nature, as well as the limited number of cases, without a significant percentage of stage IV disease. However, due to the rarity of primary SBAs and the limited targeted therapies against advanced SBAs, information derived from this investigation might be considered in future clinical trials with CLDN18-directed drugs enrolling SBA patients.
In conclusion, we found that 28% and 6% of SBAs, mainly sporadic or CrD-related, expressed CLDN18 in ≥1% (of any intensity) and ≥75% (score 2+/3+) of tumour cells, respectively, suggesting that CLDN18 may be a potential therapeutic target even in a fraction of SBAs, and that MUC5AC+/CK7+ SBAs harbour the highest probability to exhibit immunoreactivity for CLDN18.
Data availability
The datasets generated during and/or analysed during the current study are available from the corresponding author on reasonable request.
References
Pedersen KS, Raghav K, Overman MJ (2019) Small bowel adenocarcinoma: etiology, presentation, and molecular alterations. J Natl Compr Canc Netw 17(9):1135–1141. https://doi.org/10.6004/jnccn.2019.7344
Bouvier AM, Robaszkiewicz M, Jooste V, Cariou M, Drouillard A, Bouvier V, Nousbaum JB, French Network of Cancer Registries (FRANCIM) (2020) Trends in incidence of small bowel cancer according to histology: a population-based study. J Gastroenterol 55(2):181–188. https://doi.org/10.1007/s00535-019-01636-z
Legué LM, Bernards N, Gerritse SL, van Oudheusden TR, de Hingh IH, Creemers GM, Ten Tije AJ, Lemmens VE (2016) Trends in incidence, treatment and survival of small bowel adenocarcinomas between 1999 and 2013: a population-based study in The Netherlands. Acta Oncol 55(9–10):1183–1189. https://doi.org/10.1080/0284186X.2016.1182211
Aparicio T, Zaanan A, Svrcek M, Laurent-Puig P, Carrere N, Manfredi S, Locher C, Afchain P (2014) Small bowel adenocarcinoma: epidemiology, risk factors, diagnosis and treatment. Dig Liver Dis 46(2):97–104. https://doi.org/10.1016/j.dld.2013.04.013
Holmes GK, Prior P, Lane MR, Pope D, Allan RN (1989) Malignancy in coeliac disease—effect of a gluten free diet. Gut 30(3):333–338. https://doi.org/10.1136/gut.30.3.333
Bernstein CN, Blanchard JF, Kliewer E, Wajda A (2001) Cancer risk in patients with inflammatory bowel disease: a population-based study. Cancer 91:854–862. https://doi.org/10.1002/1097-0142(20010215)91:4%3c854::aid-cncr1073%3e3.0.co;2-z
Vanoli A, Di Sabatino A, Furlan D, Klersy C, Grillo F, Fiocca R, Mescoli C, Rugge M, Nesi G, Fociani P, Sampietro G, Ardizzone S, Luinetti O, Calabrò A, Tonelli F, Volta U, Santini D, Caio G, Giuffrida P, Elli L, Ferrero S, Latella G, Ciardi A, Caronna R, Solina G, Rizzo A, Ciacci C, D’Armiento FP, Salemme M, Villanacci V, Cannizzaro R, Canzonieri V, Reggiani Bonetti L, Biancone L, Monteleone G, Orlandi A, Santeusanio G, Macciomei MC, D’Incà R, Perfetti V, Sandri G, Silano M, Florena AM, Giannone AG, Papi C, Coppola L, Usai P, Maccioni A, Astegiano M, Migliora P, Manca R, Martino M, Trapani D, Cerutti R, Alberizzi P, Riboni R, Sessa F, Paulli M, Solcia E, Corazza GR, Small bowel carcinomas in coeliac or Crohn’s disease: clinico-pathological, molecular, and prognostic features (2017) A study from the Small Bowel Cancer Italian Consortium. J Crohns Colitis 11(8):942–953. https://doi.org/10.1093/ecco-jcc/jjx031
Vanoli A, Di Sabatino A, Martino M, Klersy C, Grillo F, Mescoli C, Nesi G, Volta U, Fornino D, Luinetti O, Fociani P, Villanacci V, D’Armiento FP, Cannizzaro R, Latella G, Ciacci C, Biancone L, Paulli M, Sessa F, Rugge M, Fiocca R, Corazza GR, Solcia E (2017) Small bowel carcinomas in celiac or Crohn’s disease: distinctive histophenotypic, molecular and histogenetic patterns. Mod Pathol 30(10):1453–1466. https://doi.org/10.1038/modpathol.2017.40
Surveillance, Epidemiology, and End Results (SEER) Program [Internet]. SEER*Stat Database: Incidence—SEER Research Data. https://seer.cancer.gov/statfacts/html/smint.html. Accessed January 12, 2022
Dabaja BS, Suki D, Pro B, Bonnen M, Ajani J (2004) Adenocarcinoma of the small bowel: presentation, prognostic factors, and outcome of 217 patients. Cancer 101(3):518–526. https://doi.org/10.1002/cncr.20404
Swisshelm K, Macek R, Kubbies M (2005) Role of claudins in tumorigenesis. Adv Drug Deliv Rev 57(6):919–928. https://doi.org/10.1016/j.addr.2005.01.006
Tsukita S, Tanaka H, Tamura A (2019) The claudins: from tight junctions to biological systems. Trends Biochem Sci 44(2):141–152. https://doi.org/10.1016/j.tibs.2018.09.008
Li J (2021) Context-dependent roles of claudins in tumorigenesis. Front Oncol 11:676781. https://doi.org/10.3389/fonc.2021.676781
Niimi T, Nagashima K, Ward JM, Minoo P, Zimonjic DB, Popescu NC, Kimura S (2001) Claudin-18, a novel downstream target gene for the T/EBP/NKX2.1 homeodomain transcription factor, encodes lung- and stomach-specific isoforms through alternative splicing. Mol Cell Biol 21(21):7380–90. https://doi.org/10.1128/MCB.21.21.7380-7390.2001
Karanjawala ZE, Illei PB, Ashfaq R, Infante JR, Murphy K, Pandey A, Schulick R, Winter J, Sharma R, Maitra A, Goggins M, Hruban RH (2008) New markers of pancreatic cancer identified through differential gene expression analyses: claudin 18 and annexin A8. Am J Surg Pathol 32(2):188–196. https://doi.org/10.1097/PAS.0b013e31815701f3
Sahin U, Koslowski M, Dhaene K, Usener D, Brandenburg G, Seitz G, Huber C, Türeci O (2008) Claudin-18 splice variant 2 is a pan-cancer target suitable for therapeutic antibody development. Clin Cancer Res 14(23):7624–7634. https://doi.org/10.1158/1078-0432.CCR-08-1547
Iwaya M, Hayashi H, Nakajima T, Matsuda K, Kinugawa Y, Tobe Y, Tateishi Y, Iwaya Y, Uehara T, Ota H (2021) Colitis-associated colorectal adenocarcinomas frequently express claudin 18 isoform 2: implications for claudin 182 monoclonal antibody therapy. Histopathology 79(2):227–237. https://doi.org/10.1111/his.14358
Coati I, Lotz G, Fanelli GN, Brignola S, Lanza C, Cappellesso R, Pellino A, Pucciarelli S, Spolverato G, Guzzardo V, Munari G, Zaninotto G, Scarpa M, Mastracci L, Farinati F, Realdon S, Pilati P, Lonardi S, Valeri N, Rugge M, Kiss A, Loupakis F, Fassan M (2019) Claudin-18 expression in oesophagogastric adenocarcinomas: a tissue microarray study of 523 molecularly profiled cases. Br J Cancer 121(3):257–263. https://doi.org/10.1038/s41416-019-0508-4
Morin PJ (2005) Claudin proteins in human cancer: promising new targets for diagnosis and therapy. Cancer Res 65(21):9603–9606. https://doi.org/10.1158/0008-5472
Wöll S, Schlitter AM, Dhaene K, Roller M, Esposito I, Sahin U, Türeci Ö (2014) Claudin 18.2 is a target for IMAB362 antibody in pancreatic neoplasms. Int J Cancer 134(3):731–9. https://doi.org/10.1002/ijc.28400
Lordick F, Al-Batran SE, Ganguli A, Morlock R, Sahin U, Türeci Ö (2021) Patient-reported outcomes from the phase II FAST trial of zolbetuximab plus EOX compared to EOX alone as first-line treatment of patients with metastatic CLDN18.2+ gastroesophageal adenocarcinoma. Gastric Cancer 24(3):721–730. https://doi.org/10.1007/s10120-020-01153-6
Sahin U, Türeci Ö, Manikhas G, Lordick F, Rusyn A, Vynnychenko I, Dudov A, Bazin I, Bondarenko I, Melichar B, Dhaene K, Wiechen K, Huber C, Maurus D, Arozullah A, Park JW, Schuler M, Al-Batran SE (2021) FAST: a randomised phase II study of zolbetuximab (IMAB362) plus EOX versus EOX alone for first-line treatment of advanced CLDN18.2-positive gastric and gastro-oesophageal adenocarcinoma. Ann Oncol 32(5):609–619. https://doi.org/10.1016/j.annonc.2021.02.005
Jiang H, Shi Z, Wang P, Wang C, Yang L, Du G, Zhang H, Shi B, Jia J, Li Q, Wang H, Li Z (2019) Claudin18.2-specific chimeric antigen receptor engineered T cells for the treatment of gastric cancer. J Natl Cancer Inst 111(4):409–418. https://doi.org/10.1093/jnci/djy134
Bębnowska D, Grywalska E, Niedźwiedzka-Rystwej P, Sosnowska-Pasiarska B, Smok-Kalwat J, Pasiarski M, Góźdź S, Roliński J, Polkowski W (2020) CAR-T cell therapy—an overview of targets in gastric cancer. J Clin Med 9(6):1894. https://doi.org/10.3390/jcm9061894
Sanada Y, Oue N, Mitani Y, Yoshida K, Nakayama H, Yasui W (2006) Down-regulation of the claudin-18 gene, identified through serial analysis of gene expression data analysis, in gastric cancer with an intestinal phenotype. J Pathol 208(5):633–642. https://doi.org/10.1002/path.1922
Pellino A, Brignola S, Riello E, Niero M, Murgioni S, Guido M, Nappo F, Businello G, Sbaraglia M, Bergamo F, Spolverato G, Pucciarelli S, Merigliano S, Pilati P, Cavallin F, Realdon S, Farinati F, Dei Tos AP, Zagonel V, Lonardi S, Loupakis F, Fassan M (2021) Association of CLDN18 protein expression with clinicopathological features and prognosis in advanced gastric and gastroesophageal junction adenocarcinomas. J Pers Med 11(11):1095. https://doi.org/10.3390/jpm11111095
Matsuda M, Sentani K, Noguchi T, Hinoi T, Okajima M, Matsusaki K, Sakamoto N, Anami K, Naito Y, Oue N, Yasui W (2010) Immunohistochemical analysis of colorectal cancer with gastric phenotype: claudin-18 is associated with poor prognosis. Pathol Int 60(10):673–680. https://doi.org/10.1111/j.1440-1827.2010.02587.x
Shinozaki A, Shibahara J, Noda N, Tanaka M, Aoki T, Kokudo N, Fukayama M (2011) Claudin-18 in biliary neoplasms. Its significance in the classification of intrahepatic cholangiocarcinoma. Virchows Arch 459(1):73–80. https://doi.org/10.1007/s00428-011-1092-z
Oshima T, Shan J, Okugawa T, Chen X, Hori K, Tomita T, Fukui H, Watari J, Miwa H (2013) Down-regulation of claudin-18 is associated with the proliferative and invasive potential of gastric cancer at the invasive front. PLoS ONE 8(9):e74757. https://doi.org/10.1371/journal.pone.0074757
Hagen SJ, Ang LH, Zheng Y, Karahan SN, Wu J, Wang YE, Caron TJ, Gad AP, Muthupalani S, Fox JG (2018) Loss of tight junction protein claudin 18 promotes progressive neoplasia development in mouse stomach. Gastroenterology 155(6):1852–1867. https://doi.org/10.1053/j.gastro.2018.08.041
Jovov B, Van Itallie CM, Shaheen NJ, Carson JL, Gambling TM, Anderson JM, Orlando RC (2007) Claudin-18: a dominant tight junction protein in Barrett’s esophagus and likely contributor to its acid resistance. Am J Physiol Gastrointest Liver Physiol 293(6):G1106–G1113. https://doi.org/10.1152/ajpgi.00158.2007
Tanaka M, Shibahara J, Fukushima N, Shinozaki A, Umeda M, Ishikawa S, Kokudo N, Fukayama M (2011) Claudin-18 is an early-stage marker of pancreatic carcinogenesis. J Histochem Cytochem 59(10):942–952. https://doi.org/10.1369/0022155411420569
Arpa G, Vanoli A, Grillo F, Fiocca R, Klersy C, Furlan D, Sessa F, Ardizzone S, Sampietro G, Macciomei MC, Nesi G, Tonelli F, Capella C, Latella G, Ciardi A, Caronna R, Lenti MV, Ciccocioppo R, Barresi V, Malvi D, D’Errico A, Rizzello F, Poggioli G, Mescoli C, Rugge M, Luinetti O, Paulli M, Di Sabatino A, Solcia E (2021) Prognostic relevance and putative histogenetic role of cytokeratin 7 and MUC5AC expression in Crohn’s disease-associated small bowel carcinoma. Virchows Arch 479(4):667–678. https://doi.org/10.1007/s00428-021-03109-2
Xue Y, Vanoli A, Balci S, Reid MM, Saka B, Bagci P, Memis B, Choi H, Ohike N, Tajiri T, Muraki T, Quigley B, El-Rayes BF, Shaib W, Kooby D, Sarmiento J, Maithel SK, Knight JH, Goodman M, Krasinskas AM, Adsay V (2017) Non-ampullary-duodenal carcinomas: clinicopathologic analysis of 47 cases and comparison with ampullary and pancreatic adenocarcinomas. Mod Pathol 30(2):255–266. https://doi.org/10.1038/modpathol.2016.174
Amin MB, Gress DM (2017) AJCC cancer staging manual, 8th edn. Springer, New York
Vanoli A, Guerini C, Grillo F, Klersy C, Fassan M, Arpa G, Neri G, Luinetti O, Lenti MV, Ulivi P, Tedaldi G, Furlan D, Quaquarini E, Ardizzone S, Sampietro G, Biancone L, Monteleone G, Solcia E, Sessa F, Paulli M, Adsay NV, Di Sabatino A (2022) Poorly cohesive carcinoma of the nonampullary small intestine: a distinct histologic subtype with prognostic significance. Am J Surg Pathol 46(4):498–508. https://doi.org/10.1097/PAS.0000000000001821
Pereira D, Kővári B, Brown I, Chaves P, Choi WT, Clauditz T, Ghayouri M, Jiang K, Miller GC, Nakanishi Y, Kim KM, Kim BH, Kumarasinghe MP, Kushima R, Ushiku T, Yozu M, Srivastava A, Goldblum JR, Pai RK, Lauwers GY (2021) Non-conventional dysplasias of the tubular gut: a review and illustration of their histomorphological spectrum. Histopathology 78(5):658–675. https://doi.org/10.1111/his.14294
Dottermusch M, Krüger S, Behrens HM, Halske C, Röcken C (2019) Expression of the potential therapeutic target claudin-18.2 is frequently decreased in gastric cancer: results from a large Caucasian cohort study. Virchows Arch 475(5):563–571. https://doi.org/10.1007/s00428-019-02624-7
Giuffrida P, Arpa G, Grillo F, Klersy C, Sampietro G, Ardizzone S, Fociani P, Fiocca R, Latella G, Sessa F, D’Errico A, Malvi D, Mescoli C, Rugge M, Nesi G, Ferrero S, Furlan D, Poggioli G, Rizzello F, Macciomei MC, Santini D, Volta U, De Giorgio R, Caio G, Calabrò A, Ciacci C, D’Armiento M, Rizzo A, Solina G, Martino M, Tonelli F, Villanacci V, Cannizzaro R, Canzonieri V, Florena AM, Biancone L, Monteleone G, Caronna R, Ciardi A, Elli L, Caprioli F, Vecchi M, D’Incà R, Zingone F, D’Odorico A, Lenti MV, Oreggia B, Reggiani Bonetti L, Astegiano M, Biletta E, Cantoro L, Giannone AG, Orlandi A, Papi C, Perfetti V, Quaquarini E, Sandri G, Silano M, Usai P, Barresi V, Ciccocioppo R, Luinetti O, Pedrazzoli P, Pietrabissa A, Viglio A, Paulli M, Corazza GR, Solcia E, Vanoli A, Di Sabatino A (2020) PD-L1 in small bowel adenocarcinoma is associated with etiology and tumor-infiltrating lymphocytes, in addition to microsatellite instability. Mod Pathol 33(7):1398–1409. https://doi.org/10.1038/s41379-020-0497-0
Arpa G, Grillo F, Giuffrida P, Nesi G, Klersy C, Mescoli C, Lenti MV, Lobascio G, Martino M, Latella G, Malvi D, Macciomei MC, Fociani P, Villanacci V, Rizzo A, Ferrero S, Sessa F, Orlandi A, Monteleone G, Biancone L, Cantoro L, Tonelli F, Ciardi A, Poggioli G, Rizzello F, Ardizzone S, Sampietro G, Solina G, Oreggia B, Papi C, D’Incà R, Vecchi M, Caprioli F, Caronna R, D’Errico A, Fiocca R, Rugge M, Corazza GR, Luinetti O, Paulli M, Solcia E, Di Sabatino A, Vanoli A (2020) Separation of low- versus high-grade Crohn’s disease-associated small bowel carcinomas is improved by invasive front prognostic marker analysis. J Crohns Colitis 14(3):295–302. https://doi.org/10.1093/ecco-jcc/jjz140
Vanoli A, Grillo F, Guerini C, Neri G, Arpa G, Klersy C, Nesi G, Giuffrida P, Sampietro G, Ardizzone S, Fociani P, Fiocca R, Latella G, Sessa F, D’Errico A, Malvi D, Mescoli C, Rugge M, Ferrero S, Poggioli G, Rizzello F, Macciomei MC, Santini D, Volta U, De Giorgio R, Caio G, Calabrò A, Ciacci C, D’Armiento M, Rizzo A, Solina G, Martino M, Tonelli F, Villanacci V, Cannizzaro R, Canzonieri V, Florena AM, Biancone L, Monteleone G, Caronna R, Ciardi A, Elli L, Caprioli F, Vecchi M, D’Incà R, Zingone F, D’Odorico A, Lenti MV, Oreggia B, Reggiani Bonetti L, Giannone AG, Orlandi A, Barresi V, Ciccocioppo R, Amodeo G, Biletta E, Luinetti O, Pedrazzoli P, Pietrabissa A, Corazza GR, Solcia E, Paulli M, Di Sabatino A (2021) Prognostic role of mismatch repair status, histotype and high-risk pathologic features in stage II small bowel adenocarcinomas. Ann Surg Oncol 28(2):1167–1177. https://doi.org/10.1245/s10434-020-08926-4
Jun KH, Kim JH, Jung JH, Choi HJ, Chin HM (2014) Expression of claudin-7 and loss of claudin-18 correlate with poor prognosis in gastric cancer. Int J Surg 12(2):156–162. https://doi.org/10.1016/j.ijsu.2013.11.022
Suzuki K, Sentani K, Tanaka H, Yano T, Suzuki K, Oshima M, Yasui W, Tamura A, Tsukita S (2019) Deficiency of stomach-type claudin-18 in mice induces gastric tumor formation independent of H pylori infection. Cell Mol Gastroenterol Hepatol 8(1):119–142. https://doi.org/10.1016/j.jcmgh.2019.03.003
Dotti I, Mora-Buch R, Ferrer-Picón E, Planell N, Jung P, Masamunt MC, Leal RF, Martín de Carpi J, Llach J, Ordás I, Batlle E, Panés J, Salas A (2017) Alterations in the epithelial stem cell compartment could contribute to permanent changes in the mucosa of patients with ulcerative colitis. Gut 66(12):2069–2079. https://doi.org/10.1136/gutjnl-2016-312609
Zwiers A, Fuss IJ, Leijen S, Mulder CJ, Kraal G, Bouma G (2008) Increased expression of the tight junction molecule claudin-18 A1 in both experimental colitis and ulcerative colitis. Inflamm Bowel Dis 14(12):1652–1659. https://doi.org/10.1002/ibd.20695
Satake S, Semba S, Matsuda Y, Usami Y, Chiba H, Sawada N, Kasuga M, Yokozaki H (2008) Cdx2 transcription factor regulates claudin-3 and claudin-4 expression during intestinal differentiation of gastric carcinoma. Pathol Int 58(3):156–163. https://doi.org/10.1111/j.1440-1827.2007.02204.x
Arnold A, Daum S, von Winterfeld M, Berg E, Hummel M, Rau B, Stein U, Treese C (2020) Prognostic impact of Claudin 18.2 in gastric and esophageal adenocarcinomas. Clin Transl Oncol 22(12):2357–2363. https://doi.org/10.1007/s12094-020-02380-0
Ungureanu BS, Lungulescu CV, Pirici D, Turcu-Stiolica A, Gheonea DI, Sacerdotianu VM, Liliac IM, Moraru E, Bende F, Saftoiu A (2021) Clinicopathologic relevance of claudin 18.2 expression in gastric cancer: a meta-analysis. Front Oncol 11:643872. https://doi.org/10.3389/fonc.2021.643872
Acknowledgements
We thank all the Small Bowel Cancer Italian Consortium Collaborators for their support.
Funding
Open access funding provided by Università degli Studi di Pavia within the CRUI-CARE Agreement. This work was supported by Fondazione IRCCS (Istituto di Ricovero e Cura a Carattere Scientifico) San Matteo Hospital [Ministero Italiano della Salute]. MF is supported by grants from the Italian Health Ministry/Veneto region research program NET-2016–02363853 and AIRC 5 per mille 2019 (ID. 22759 program). The funding agencies had no role in the design and performance of the study.
Author information
Authors and Affiliations
Contributions
Concept and design the study: GA, MF, CG, AV. Acquisition of data, or analysis and interpretation of data: GA, MF, CG, EQ, FG, VA, VG, SL, FB, MVL, PP, MP, ADS and AV. Drafting the article: GA, CG and AV. Revising the manuscript critically for important intellectual content: MF, EQ, FG, VA, VG, SL, FB, MVL, PP, MP, ADS and AV. Final approval of the version submitted: GA, MF, CG, EQ, FG, VA, VG, SL, FB, MVL, PP, MP, ADS and AV. GA, MF, CG, EQ, FG, VA, VG, SL, FB, MVL, PP, MP, ADS and AV agree to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
This study was approved by the Ethics Committee of Pavia (protocol number: 20140003980). Informed consent was obtained for all patients enrolled in the study.
Conflict of interest
Matteo Fassan reports personal fees (as speaker bureau or advisor) from Roche, MSD, GSK, Astellas Pharma, Diaceutics and received research grants from Astellas Pharma, QED therapeutics and macrophage pharma, unrelated to the current work. Giovanni Arpa, Camilla Guerini, Erica Quaquarini, Federica Grillo, Valentina Angerilli, Vincenza Guzzardo, Sara Lonardi, Francesca Bergamo, Marco Vincenzo Lenti, Paolo Pedrazzoli, Marco Paulli, Antonio Di Sabatino and Alessandro Vanoli have no financial or non-financial interests to declare that are relevant to the content of this article.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Giovanni Arpa and Matteo Fassan are co-first authors
Antonio Di Sabatino and Alessandro Vanoli are co-last authors
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/
About this article
Cite this article
Arpa, G., Fassan, M., Guerini, C. et al. Claudin-18 expression in small bowel adenocarcinoma: a clinico-pathologic study. Virchows Arch 481, 853–863 (2022). https://doi.org/10.1007/s00428-022-03393-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00428-022-03393-6