Abstract
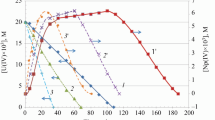
The oxidation-reduction reaction between U(VI) and Ti(III) in HCl solution was studied spectrophotometrically. The reaction is second-order at all concentrations of reactants, HCl, ferrous chloride and mannitol used in this work. In 5M HCl the rate constantk increases with increasing Ti(III) concentration, whereas it decreases with increasing U(VI) concentration, with increasing HCl concentration from 1.00M to 7.17M and increases thereafter from 7.17M to 11.79M. The addition of mannitol causes a consistent decrease in the rate of reaction, whereas ferrous chloride has no effect. The activation energy for this oxidation-reduction reaction was 47.90±0.11 kJ·mol−1. The values of ΔH ≠, ΔG ≠ and ΔS ≠ were 45.40±0.11 kJ·mol−1, 72.50±0.17 kJ·mol−1 and −91.10±0.22J·k−1·mol−1, respectively. The mode of reaction is discussed in the light of kinetic results.
Similar content being viewed by others
References
T. W. NEWTON, The kinetics of the oxidation-reduction reactions of uranium, neptunium, plutonium and americium in aqueous solutions, Report TID 26506. Published by Technical Information Center, Office of Public Affairs, U.S. Energy Research and Development Administration, 1975.
T. P. LOGAN, J. P. BIRK, Inorg. Chem., 12 (1973) 2464.
S. W. RABIDEAU, R. J. KLINE, J. Inorg. Nucl. Chem., 14 (1960) 91.
R. H. BETTS, Can. J. Chem., 33 (1955) 1780.
J. C. SULLIVAN, R. C. THOMPSON, Inorg. Chem., 6 (1967) 1795.
E. H. APPELMAN, J. C. SULLIVAN, J. Phys. Chem., 66 (1962) 442.
T. W. NEWTON, F. B. BAKER, J. Phys. Chem. 69 (1965) 176.
T. W. NEWTON, J. Phys. Chem., 62 (1958) 943.
J. H. ESPENSON, R. T. WANG, Inorg. Chem., 11 (1972) 955.
V. I. SPITSYN, J. Inorg. Nucl. Chem., 31 (1969) 2733.
H. L. TUCKER, R. McELHANEY, Determination of uranium in uranium metal, uranium oxides and uranyl nitrate solution by potentiometric titration; Oak Ridge, Y-12 plant, YIDK-345, DE 83013565, 10th Annual Conf. of National Organization of Black Chemists and Chemical Engineers, Knoxville, Tennessee, May, 25–28. 1983.
T. L. WILLIAMS, G. M. TUCKER, G. A. HUFF, L. G. JORDAN, Determination of free acid in plutonium(IV) solutions thermometrically, potentiometrically; Analytical Chemistry in Nuclear Technology, W. S. LYON (Ed.), Ann Arbor-Science, 1982, p. 97.
E. W. BAUMANN, Standard addition method for free acid determination in solutions with hydrolyzable ions; Analytical Chemistry in Nuclear Technology, W. S. LYON (Ed.), Ann Arbor-Science, 1982, p. 105.
O. JOHNSON, Report CC-1524 (A-2202) May, 1944.
J. J. KATZ, E. RABINOWITCH (Eds), Chemistry of U (collected papers), U.S. Atomic Energy Commission, Technical Information Service, Extension, Oak Ridge, Tennessee 1958.
A. I. VOGEL, A Textbook of Quantitative Inorganic Analysis, 3rd ed., The English Language Book Society and Longmans Green and Co. Ltd., 1964, Chapter 4.
Vogel's Textbook of Quantitative Inorganic Analysis Including Elementary Instrumental Analysis. 4th ed; edited and revised by J. BASSETT et al., The English Language Book Society and Longmans Green and Co. Ltd., 1978, p. 360.
J. H. ESPENSON, R. T. WANG, Chem. Comm., 207 (1970).
S. MAOMI, I. KOHJI, M. TETSUYA, O. KAZUSHI, S. TOMIO, Asahi Chemical Industry Co. Ltd., Osaka, Japan, Process for separating uranium isotopes, Brit. Patent 1,565,392(CL.B01D59/00), 23 April 1980, Appl. 76/33,587, 12 August 1976, p. 18.
T. MIYAKE, M. SEKO, K. INADA, K. OCHI, T. SAKAMOTO, Asahi Chemical Industry Co. Ltd., Osaka, Japan, Australian Patent document 506772/B./Int. CL B01D59/30. 1. Dec. 1977. VP. Filed 24 May 1976, accepted 24 Jan. 1980, Priority 6 Sep. 1975 (JP 108348/75).
G. GORDON, H. J. TAUBE, J. Inorg. Nucl. Chem., 16 (1961) 272.
G. GORDON, H. J. TAUBE J. Inorg. Nucl. Chem., 17 (1962) 69.
R. D. CANNON, Electron Transfer Reactions, Butterworths, London, 1980, p. 53.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Hamid, M.A., Rehman, E.U., Fuzail, M. et al. The kinetics of the oxidation-reduction reaction between uranium(VI) and titanium(III) in HCl solution. Journal of Radioanalytical and Nuclear Chemistry, Articles 190, 37–50 (1995). https://doi.org/10.1007/BF02035635
Received:
Issue Date:
DOI: https://doi.org/10.1007/BF02035635