Summary
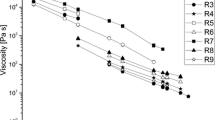
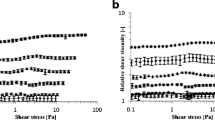
The rheological properties of a sodium tallow-coconut oil soap (15% water) have been determined using a high pressure capillary extrusion viscometer over shear rates of 14.7 to 2560 sec−1 and temperatures of 70–103°C. Capillary flow measurements were also made on sodium stearate (25% water) at 90°C. The data indicated shear thinning characteristics and were fitted to an equation of the form:
over the above shear rate range. The flow indices (n) of 0.337–0.437 were comparable to those obtained from polyethylene data in the literature. A zero shear activation energy of 57.2 Kcal/mole was calculated for the tallow-coconut soap. The activation energy at constant shear stress was greater than that at constant shear rate and decreased with increasing shear stress and shear rate. The soap flow unit was estimated to contain about 2 · 104 molecules.
Similar content being viewed by others
References
Southam, F. W. andI. E. Puddington, Can. J. Research25 B, 125 (1947).
Powell, B. D. andI. E. Puddington, Can. J. Chem.31, 828 (1953).
Vold, R. D. andL. L. Lyon, Ind. Eng. Chem.37, 497 (1945).
Lyon, L. L. andR. D. Vold, Ind. Eng. Chem. Anal. Ed.17, 585 (1945).
Sekiguchi, H. andY. Kimura, Yukagaku9, 399 (1960).
Bujake, J. E., Jr., Rev. Sci. Instrum.36, 1368 (1965).
Davidsohn, J., E. J. Better andA. Davidsohn, Soap Manufacture, Vol. 1, p. 371 (New York 1953).
Bagley, E. B., J. Appl. Phys.28, 624 (1957).
Rabinowitsch, B., Z. Physik. Chem. (Leipzig)145 A, 1 (1929).
Metzger, A. P. andJ. R. Knox, Trans. Soc. Rheol.9, 13 (1965).
Toor, H. L., Trans. Soc. Rheol.1, 177 (1957).
Meissner, J., Proc. of Fourth International Congress on Rheology,E. H. Lee, ed., Part. 3, p. 437 (New York 1965).
Schott, H. andW. S. Kaghan, J. Appl. Polymer Sci.5, 175 (1961).
Smallwood, H. M., J. Appl. Phys.8, 505 (1937).
Bestul, A. B. andH. V. Belcher, J. Appl. Phys.24, 696 (1953).
Philippoff, W. andF. H. Gaskins, J. Polymer Sci.21, 205 (1956).
Marton, L., J. W. McBain, andR. D. Vold, J. Amer. Chem. Soc.63, 1990 (1941).
Chwalow, M., J. Phys. Chem.57, 354 (1953).
Shuttleworth, T. H. andM. Camp, Nature183, 535 (1959).
Segerman, E., Acta Cryst.19, 789 (1965).
McBain, J. W. andW. W. Lee, Oil & Soap20, 17 (1943).
Luzzati, V., H. Mustacchi andA. Skoulis, Nature180, 600 (1957).
Buerger, M. J., L. B. Smith, F. V. Ryer, andJ. E. Spike, Jr., Proc. Nat. Acad. Sci.31, 226 (1945).
Glasstone, S., K. J. Laidler andH. Eyring, Theory of Rate Processes p. 513–514 (New York, 1941).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Bujake, J.E. Capillary viscometry of sodium soaps. Rheol Acta 5, 227–232 (1966). https://doi.org/10.1007/BF01982432
Received:
Issue Date:
DOI: https://doi.org/10.1007/BF01982432