Abstract
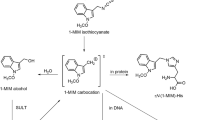
Using32P-postlabelling, we examined DNA binding by 2,4 and 2,6-dinitrotoluene (DNT) in Fischer-344 rats. DNA binding between the two compounds was compared to determine if differences in adduct formation and persistence could partly explain the known isomerspecific hepatocarcinogenicity of DNTs. The differences in cytotoxicity between the two isomers were also assessed. Both 2,4 and 2,6-DNT induced adduct formation in hepatic DNA. Three distinct adducts were detected following single i.p. administration of 2,4-DNT, while the 2,6-isomer produced four different adducts. Depending on the concentration administered, the two compounds differed in their relative yields. 2,6-DNT produced a greater total adduct yield relative to the 2,4-isomer at low concentrations. Following administration of high concentrations, however, 2,4-DNT predominated. The maximum adduct levels measured were 3.0 and 1.8 adducted nucleotides per 106 nucleotides for 2,4 and 2,6-DNT, respectively. Substantial amounts of adducts from both compounds were found to persist over time. After 2 weeks, the mean persistence for 2,4 and 2,6-DNT induced adducts were 42% and 46%, respectively. Qualitative examination for liver toxicity showed 2,6-DNT to be more cytotoxic, inducing extensive hemorrhagic centrilobular necrosis. Rats treated with 2,4-DNT did not show any observable signs of hepatocellular necrosis. Under the conditions of this study, the differences between 2,4 and 2,6-DNT in adduct formation and persistence do not appear to be sufficient to account for their differences in carcinogenicity. The toxicity of 2,6-DNT may be a determining factor in the potent carcinogenicity observed with this compound.
Similar content being viewed by others
References
Brookes P, Lawley PD (1964) Evidence for the binding of polynuclear aromatic hydrocarbons to the nucleic acids of mouse skin: relation between carcinogenic power of hydrocarbons and their binding to DNA. Nature 202: 781–784
Cayama E, Tsuda H, Sarma DSR, Farber E (1978) Initiation of chemical carcinogenesis requires cell proliferation. Nature 275: 60–62
Chemical Industry Institute of Toxicology (1982) 104-Week chronic toxicity study in rats. Dinitrotoluene. Final Report. Vol. 1, CIIT Docket No. 12 362, CIIT, Research Triangle Park, NC
Columbano A, Rajalakshmi S, Sarma DSR (1981) Requirement of cell proliferation for the initiation of liver carcinogenesis as assayed by three different procedures. Cancer Res 41: 2079–2083
Dixit R, Schut HAJ, Klaunig JE, Stoner GD (1986) Metabolism and DNA binding of 2,6-dinitrotoluene in Fischer-344 rats and A/J mice. Toxicol Appl Pharmacol 82: 53–61
Ellis HV, Hagensen JH, Hodgson JR, Minor JL, Hong C-B, Ellis ER, Girvin JD, Helton DO, Herndon BL, Lee C-C (1979) Mammalian toxicity of munition compounds. Phase III: effects of life-time exposure. Part 1: 2,4-dinitrotoluene. Final report No. 7, Midwest Research Institute Project No. 3900-B, Midwest Research Institute, Kansas City, MO
Gupta RC (1984) Nonrandom binding of the carcinogen N-hydroxy-2-acetylaminofluorene to repetitive sequences of rat liver DNA in vivo. Proc Natl Acad Sci 81: 6943–6947
Hemminki K (1983) Nucleic acid adducts of chemical carcinogens and mutagens. Arch Toxicol 52: 249–285
Kaplan MM (1987) Laboratory tests. In: Schiff L, Schiff ER (eds) Diseases of the liver. J. B. Lippincott Co., Philadelphia, PA, pp 219–260
Kedderis GL, Dyroff MC, Rickert DE (1984) Hepatic macromolecular covalent binding of the hepatocarcinogen 2,6-dinitrotoluene and its 2,4-isomer in vivo: modulation by the sulfotransferase inhibitors pentachlorophenol and 2,6-dichloro-4-nitrophenol. Carcinogenesis 5: 1199–1204
Leonard TB, Lyght O, Popp JA (1983) Dinitrotoluene structure-dependent initiation of hepatocytes in vivo. Carcinogenesis 4: 1059–1061
Leonard TB, Adams T, Popp JA (1986) Dinitrotoluene isomer-specific enhancement of the expression of diethylnitrosamine-initiated hepatocyte foci. Carcinogenesis 7: 1797–1803
Leonard TB, Graichen ME, Popp JA (1987) Dinitrotoluene isomer-specific hepatocarcinogenesis in F344 rats. JNCI 79: 1313–1319
Lutz WK (1979) In vivo covalent binding of organic chemicals to DNA as a quantitative indicator in the process of chemical carcinogenesis. Mutat Res 65: 289–356
Martin CN, Ekers SF (1980) Studies on the macromolecular binding of benzidine. Carcinogenesis 1: 101–109
Miller EC (1978) Some current prespectives on chemical carcinogenesis in humans and experimental animals: presidential address. Cancer Res 38: 1479–1496
Mirsalis JC, Butterworth BE (1982) Induction of unscheduled DNA synthesis in rat hepatocytes following in vivo treatment with dinitrotoluene. Carcinogenesis 3: 241–245
Nakayama J, Yuspa SH, Poirier MC (1984) Benzo(a)pyrene-DNA adduct formation and removal in mouse epidermis in vivo and in vitro: relationship of DNA binding to initiation of skin carcinogenesis. Cancer Res 44: 4087–4095
NationalCancer Institute (1978) Bioassay of 2,4-dinitrotoluene for possible carcinogenicity. National Cancer Institute Carcinogenesis Technical Report Series, No. 54, DHEW Publication No. (NIH) 78-1360, NCI, National Institutes of Health, Bethesda, MD
Phillips DH, Grover PL, Sims P (1979) A quantitative determination of the covalent binding of a series of polycyclic hydrocarbons to DNA in mouse skin. Int J Cancer 23: 201–208
Phillips DH, Miller JA, Miller EC, Adams B (1981) N2 atom of guanine and N6 atom of adenine residues as sites for covalent binding of metabolically activated 1′-hydroxysafrole to mouse liver DNA in vivo. Cancer Res 41: 2664–2671
Poirier MC, Hunt JM, True BA, Laishes BA, Young JF, Beland FA (1984) DNA adduct formation, removal and persistence in rat liver during one month of feeding 2-acetylaminofluorene. Carcinogenesis 5: 1591–1596
Randerath K, Haglund RE, Phillips DH, Reddy MV (1984)32P-Postlabelling analysis of DNA adducts formed in the livers of animals treated with safrole, estragole and other naturally-occurring alkenylbenzenes. I. Adult female CD-1 mice. Carcinogenesis 5: 1613–1622
Reddy MV, Randerath K (1986) Nuclease P1-mediated enhancement of sensitivity of32P-postlabeling test for structurally diverse DNA adducts. Carcinogenesis 7: 1543–1551
Rickert DE, Schnell SR, Long RM (1983) Hepatic macromolecular covalent binding and intestinal dispositon of [14C] dinitrotoluenes. J Toxicol Environ Health 11: 555–567
Ross J, Nelson G, Kligerman A, Erexson G, Bryant M, Earley K, Gupta R, Nesnow S (1990) Formation and persistence of novel benzo(a)pyrene adducts in rat lung, liver, and peripheral blood lymphocyte DNA. Cancer Res 50: 5088–5094
Singer B (1975) The chemical effects of nucleic acid alkylation and their relation to mutagenesis and carcinogenesis. Prog Nucl Acid Res Mol Biol 16: 219–284
Singer B, Essigmann JM (1991) Site-specific mutagenesis: retrospective and prospective. Carcinogenesis 12: 949–955
Swenberg JA, Dyroff MC, Bedell MA, Popp JA, Huh N, Kirstein U, Rajewsky MF (1984) O4-Ethyldeoxythymidine, but not O6-ethyldeoxyguanosine, accumulates in hepatocyte DNA of rats exposed continuously to diethylnitrosamine. Proc Natl Acad Sci 81: 1692–1695
Talaska G, Au WW, Ward Jr JB, Randerath K, Legator MS (1987) The correlation between DNA adduets and chromosomal aberrations in the target organ of benzidine exposed, partially-hepactectomized mice. Carcinogenesis 8: 1899–1905
Taningher M, Saccomanno G, Santi L, Grilli S, Parodi S (1990) Quantitative predictability of carcinogenicity of the covalent binding index of chemicals to DNA: comparison of the in vivo and in vitro assays. Environ Health Perspect 84: 183–192
Wogan GN, Gorelick NJ (1985) Chemical and biochemical dosimetry of exposure to genotoxic chemicals. Environ Health Perspect 62: 5–18
Ying TS, Sarma DSR, Farber E (1981) Role of acute hepatic necrosis in the induction of early steps in liver carcinogenesis by dimehtylnitrosamine. Cancer Res 41: 2096–2102
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
La, D.K., Froines, J.R. Comparison of DNA adduct formation between 2,4 and 2,6-dinitrotoluene by32P-postlabelling analysis. Arch Toxicol 66, 633–640 (1992). https://doi.org/10.1007/BF01981502
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF01981502