Abstract
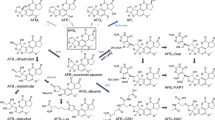
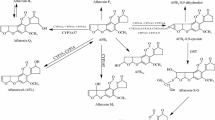
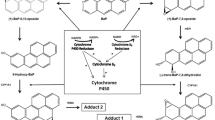
In the present study, we examined the effects of treating guinea pigs with β-naphthoflavone (BNF) on aflatoxin B1 (AFB1) metabolism by microsomal cytochrome P450 monooxygenase (P450) and prostaglandin H synthase (PHS) in liver, lung and kidney tissues. After BNF treatment, microsomal 7-ethoxyresorufin 0-deethylase activity was induced 13-, 25- and 11-fold in lung, kidney and liver, respectively, confirming that the BNF treatment protocol was effective at inducing monooxygenase activity. Treatment of guinea pigs with BNF did not change [3H]AFB1-DNA binding catalyzed by microsomal PHS or P450 in lung, kidney or liver. In contrast, AFM1 formation by P450 was significantly increased in microsomes from all three organs. The data indicate that BNF-inducible P450 isozymes of the P4501A class are responsible for the biotransformation of AFB1 to non-toxic metabolites. Guinea pig kidney microsomes could also catalyze NADPH-dependent formation of aflatoxicol (AFL), a metabolite usually produced by a cytosolic steroid dehydrogenase. Renal microsomal AFL formation was not altered by prior BNF treatment. The results in the present study suggest that BNF may alter the bioactivation of AFB1 in guinea pig tissues.by inducing P450 activity, leading to the formation of less reactive metabolite.
Similar content being viewed by others
Abbreviations
- AA:
-
arachidonic acid
- AF:
-
aflatoxin
- AFL:
-
aflatoxicol
- BNF:
-
β-naphthoflavone
- HPLC:
-
high performance liquid chromatography
- P450:
-
cytochrome P450 polysubstrate monooxygenase
- PHS:
-
prostaglandin H synthase
- PAH:
-
polycyclic aromatic hydrocarbon(s)
References
Adams RPL, Knowler JT, Leader DR (1986) The biochemistry of nucleic acids, 10th edn. Chapman and Hall, New York, NY, pp 459–462
Aoshima Y, Kochakian CD (1963) Activity, intracellular distribution and some properties of 17β-hydroxy-C19-steroid dehydrogenases in liver and kidney. Endocrinology 72: 106–114
Bailey GS, Williams DE, Wilcox JS, Loveland PM, Coulombe RA, Hendricks JD (1988) Aflatoxin B1 carcinogenesis and its relation to DNA adduct formation and adduct persistence in sensitive and resistant salmonid fish. Carcinogenesis 9: 1919–1926
Battista JR, Marnett LJ (1985) Prostaglandin H synthase-dependent epoxidation of aflatoxin B1. Carcinogenesis 6: 1227–1229
Boyd JA, Eling TE (1987) Prostaglandin H synthase-catalyzed metabolism and DNA binding of 2-naphthylamine. Cancer Res 47: 4007–4014
Bressac B, Kew M, Wands J, Ozturk M (1991) Selective G to T mutations of p53 gene in hepatocellular carcinoma from Southern Africa. Nature 350: 429–431
Burke MD, Thompson S, Elcombe CR, Halpert J, Haaparanta T, Mayer RT (1985) Ethoxy-, pentoxy- and benzyloxyphenoxazones and homologues: a series of substrates to distinguish between different induced cytochromes P-450. Biochem Pharmacol 34: 3337–3345
Daniels JM, Massey TE (1992) Modulation of aflatoxin B1 biotransformation in rabbit pulmonary and hepatic microsomes. Toxicology 74: 19–32
Daniels JM, Liu L, Stewart RK, Massey TE (1990) Biotransformation of aflatoxin B1 in rabbit lung and liver microsomes. Carcinogenesis 11: 823–827
Dickens F, Jones HEH, Waynforth HB (1966) Oral, subcutaneous and intratracheal administration of carcinogenic lactones and related substances: the intratracheal administration of cigarette tar in the rat. Br J Cancer 20: 134–144
Dvorackova I, Stora C, Ayraud N (1981) Evidence for aflatoxin B1 in two cases of lung cancer in man. J Cancer Res Clin Oncol 100: 221–224
Epstein SM, Bartus B, Farber E (1969) Renal epithelial neoplasms induced in male Wistar rats by oral aflatoxin B1. Cancer Res 29: 1045–1050
Gabliks J, Barter S (1987) Comparative cytotoxicity of aflatoxin B1 and saxitoxin in cell cultures. Mol Toxicol 1: 209–216
Garner RC, Miller EC, Miller JA, Garner JV, Hanson RS (1971) Formation of a factor lethal for S. typhimurium TA1530 and TA1531 on incubation of aflatoxin B1 with rat liver microsomes. Biochem Biophys Res Commun 45: 774–780
Garner RC, Miller EC, Miller JA (1972) Liver microsomal metabolism of aflatoxin B1 to a reactive derivative toxic toSalmonella typhimurium TA 1530. Cancer Res 32: 2058–2066
Goeger DE, Shelton DW, Hendricks JD, Pereira C, Bailey GS (1988) Comparative effect of dietary butylated hydroxyanisole and β-naphthoflavone on aflatoxin B1 metabolism, DNA adduct formation, and carcinogenesis in rainbow trout. Carcinogenesis 9: 1793–1800
Gurtoo HL, Dahms RP, Kanter P, Vaught JB (1978) Association and dissociation of the Ah Locus with the metabolism of aflatoxin B1 by mouse liver. J Biol Chem 253: 3952–3961
Hayes RB, Van Nieuwenhuize JP, Raatgever JW, ten Kate FJW (1984) Aflatoxin exposures in the industrial setting: an epidemiological study of mortality. Food Chem Toxicol 22: 39–43
Hoult JR, Bacon KB, Osborne DJ, Robinson C (1988) Organ selective conversion of prostaglandin D2 to 9 alpha, 11 beta-prostaglandin F2 and its subsequent metabolism in rat, rabbit and guinea pig. Biochem Pharmacol 37: 3591–3599
Hsu IC, Metcalf RA, Sun T, Welsh JA, Wang NJ, Harris CC (1991) Mutational hotspot in the p53 gene in human hepatocellular carcinomas. Nature 350: 427–428
Koser PL, Faletto MB, Maccubbin AE, Gurtoo HL (1988) The genetics of aflatoxin B1 metabolism. Association of the induction of aflatoxin B1-4-hydroxylase with the transcriptional activation of cytochrome P3-450 gene. J Biol Chem 263: 12584–12595
Liu L, Massey TE (1992) Bioactivation of aflatoxin B1 by lipoxygenases, prostaglandin H synthase and cytochrome P450 monooxygenase in guinea-pig tissues. Carcinogenesis 13: 533–539
Liu L, Daniels JM, Stewart RK, Massey TE (1990) In vitro prostaglandin H synthase- and monooxygenase-mediated binding of aflatoxin B1 to DNA in guinea-pig tissue microsomes. Carcinogenesis 11: 1915–1919
Lotlikar PD (1989) Metabolic basis for susceptibility and resistance to aflatoxin B1 hepatocarcinogenesis in rodents. J Toxicol Toxin Rev 8: 97–109
Loveland PM, Wilcox JS, Pawlowski NE, Bailey GS (1987) Metabolism and DNA binding of aflatoxicol and aflatoxin B1 in vivo and in isolated hepatocytes from rainbow trout(Salmo gairdneri). Carcinogenesis 8: 1065–1070
Lowry OH, Rosebrough NJ, Farr AL, Randall RJ (1951) Protein measurement with the folin phenol reagent. J Biol Chem 193: 265–275
Nebert DW (1989) The Ah locus: genetic differences in toxicity, cancer, mutation, and birth defects. CRC Crit Rev Toxicol 20: 153–174
Newberne PM, Butler WH (1969) Acute and chronic effects of aflatoxin on the liver of domestic and laboratory animals. a review. Cancer Res 29: 236–244
Oppermann UCT, Maser E, Mongoura SA, Netter KJ (1991) Heterogeneity of carbonyl reduction in subcellular fractions and different organs in rodents. Biochem Pharmacol 42: S189-S195
Patterson DSP (1973) Metabolism as a factor in determining the toxic action of the aflatoxins in different species. Food Cosmet Toxicol 11: 287–294
Patterson DSP, Roberts BA (1971) The in vitro reduction of aflatoxin B1 and B2 by soluble avian liver enzymes. Food Cosmet Toxicol 9: 829–837
Raina V, Koser P, Gurtoo HL (1985) Differential sensitivity of AH-responsive mice to β-naphthoflavone-induced metabolism of benzo[a]pyrene and aflatoxin B1. J Toxicol Environ Health 16: 255–261
Salhab AS, Edwards GS (1977) Comparative in vitro metabolism of aflatoxicol by liver preparations from animals and humans. Cancer Res 37: 1016–1021
Santhanam K, Lotlikar PD (1989) Effect of beta-naphthoflavone on the metabolism of aflatoxin B1 in hamsters. Cancer Lett 45: 129–134
Schoenhard GL, Lee DJ, Howell SE, Pawlowski NE, Libbey LM, Sinnhuber RO (1976) Aflatoxin B1 metabolism to aflatoxicol and derivatives lethal toBacillus subtilis GSY 1057 by rainbow trout (Salmo gairderi) liver. Cancer Res 36: 2040–2045
Sivarajah K, Anderson MW, Eling TE (1978) Metabolism of benzo(a)pyrene to reactive intermediates(s) via prostaglandin biosynthesis. Life Sci 23: 2571–2578
Sivarajah K, Lasker JM, Eling TE (1981) Prostaglandin synthetase-dependent cooxidation of (±)-benzo(a)pyrene-7,8-dihydrodiol by human lung and other mammalian tissues. Cancer Res 41: 1834–1839
Sivarajah K, Jones KG, Fouts JR, Devereux T, Shirley JE, Eling TE (1983) Prostaglandin synthetase and cytochrome P-450-dependent metabolism of benzo(a)pyrene 7,8-dihydrodiol by enriched populations of rat Clara cells and alveolar type II cells. Cancer Res 43: 2632–2636
Swenson DH, Miller JA, Miller EC (1973) 2,3-Dihydro-2,3-dihydroxy-aflatoxin B1: An acid hydrolysis product of an RNA-aflatoxin B1 formed by hamster and rat liver microsomes in vitro. Biochem Biophys Res Commun 53: 1260–1267
Ueno I, Friedman L, Stone CL (1980) Species difference in the binding of aflatoxin B1 to hepatic macromolecules. Toxicol Appl Pharmacol 52: 177–180
United Nations Environment Programme and World Health Organization (1979) Environmental health criteria 11: Mycotoxins, World Health Organization, Geneva
Westcott JY, McDonnell TJ, Bostwick P, Voelkel NF (1988) Eicosanoid production in isolated perfused lungs stimulated by calcium ionophore A23 187. Am Rev Respir Dis 138: 895–900
Zivin JA, Bartko JJ (1976) Statistics for disinterested scientists. Life Sci 18: 15–26
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Liu, L., Nakatsu, K. & Massey, T.E. In vitro cytochrome P450 monooxygenase and prostaglandin H-synthase mediated aflatoxin B1 biotransformation in guinea pig tissues: Effects of β-naphthoflavone treatment. Arch Toxicol 67, 379–385 (1993). https://doi.org/10.1007/BF01977398
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF01977398