Abstract
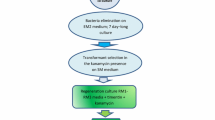
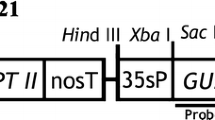
For the development of anAgrobacterium-mediated transformation procedure of carnation (Dianthus caryophyllus L.), an intron-containing β-glucuronidase (gus) gene was used to monitor the frequency of transformation events soon after infection of leaf explants. The efficiency of gene transfer was dependent on the carnation genotype, explant age and cocultivation time. Leaf explants from the youngest leaves showed the highest number of GUS-positive spots. After selection on a kanamycin-containing medium, transgenic shoots were generated among a relatively high number of untransformed shoots. The selection procedure was modified in such a way that the contact between explant and medium was more intense. This improved the selection and decreased the number of escapes. Kanamycin-resistant and GUS-positive plants were obtained from five cultivars after infection of leaf explants with the supervirulentAgrobacterium strain AGLO. A higher transformation frequency was observed with the binary vector pCGN7001 than with the p35SGUSint vector. Integration of the genes into the carnation genome was demonstrated by Southern blot hybridization. The number of incorporated T-DNA insertions varied between independent transformants from one to eight. Transformants were morphologically identical to untransformed plants. Segregation of the genes occurred in a Mendelian way.
Similar content being viewed by others
References
An, G. (1985) High efficiency transformation of cultured tobacco cells.Pl. Physiol. 79, 568–70.
Comai, L., Moran, P. and Maslyar, D. (1990) Novel and useful properties of a chimeric plant promoter combining CAMV 35S and MAS elements.Pl. Mol. Biol. 15, 373–81.
Depicker, A., Stachel, S., Dhaese, P., Zambryski, P. and Goodman, H.M. (1982) Nopaline synthase: transcript mapping and DNA sequence.J. Mol. Appl. Genet. 1, 561–73.
Feinberg, A.P. and Vogelstein, B. (1983) A technique for radiolabeling DNA restriction endonuclease fragments to high specific activity.Anal. Biochem. 132, 6–13.
Genstat 5 Committee (1988)Genstat 5 Reference Manual. Oxford: Clarendon Press.
Hood, E.E., Helmer, G.L., Fraley, R.T. and Chilton, M.D. (1986) The hypervirulence region ofAgrobacterium tumefaciens A281 is encoded in a region of pTiBO-542 outside of T-DNA.J. Bacteriol. 168, 1291–301.
Horsch, R.B., Fry, J.E., Hoffmann, N.L., Eicholtz, D., Rogers, S.G. and Fraley, R.T. (1985) A simple and general method of transferring genes into plants.Science 227, 1229–31.
Jansen, J. and Hoekstra, J.A. (1993) The analysis of proportions in agricultural experiments by a generalized linear model.Statist. Neerl. 47, 161–74.
Jefferson, R.A., Kavanagh, T.A. and Bevan, M. (1987) GUS-fusions: β-glucuronidase as a sensitive and versatile gene fusion marker in higher plants.EMBO J. 6, 3901–7.
Karp, A. and Bright, S.W.J. (1985) On the causes and origins of somaclonal variation.Oxford Surveys Pl. Mol. Cell Biol. 2, 199–234.
Koncz, C. and Schell, J. (1986) The promoter of TL-DNA gene 5 controls the tissue specific expression of chimaeric genes carried by a novel type ofAgrobacterium binary vector.Mol. Gen. Genet. 204, 383–96.
Lazo, G.R., Stein, P.A. and Ludwig, R.A. (1991) A DNA transformation-competentArabidopsis genomic library inAgrobacterium.Bio/Technology 9, 963–7.
Lu, C.Y., Nugent, G., Wardley-Richardson, T., Chandler, S.F., Young, R. and Dalling, M.J. (1991)Agrobacterium mediated transformation of carnation (Dianthus caryophyllus L.).Bio/Technology 9, 864–8.
Murashige, T. and Skoog, F. (1962) A revised medium for rapid growth and bio-assays with tobacco tissue cultures.Physiol. Plant. 15, 473–97.
Odell, J.T., Nagy, F. and Chua, N.H. (1985) Identification of DNA sequences required for activity of the cauliflower mosaic virus 35S promoter.Nature 313, 810–12.
Sanders, P.E., Winter, J.A., Barnason, A.R., Rogers, S.G. and Fraley, R.T. (1987) Comparison of the cauliflower mosaic virus 35S and nopaline synthase promoters in transgenic plants.Nucl. Acids Res. 15, 1543–58.
Sushan, S. and Johnson, N.A. (1955) The shoot apex and leaf ofDianthus caryophyllus L.Bull. Torrey Bot. Club 82, 266–83.
Vancanneyt, G., Schmidt, R., O'Connor-Sanchez, A., Willmitzer, L. and Rocha-Sosa, M. (1990) Construction of an introncontaining marker gene: splicing of the intron in transgenic plants and its use in monitoring early events inAgrobacterium mediated plant transformation.Mol. Gen. Genet. 220, 245–50.
Van Altvorst, A.C. Van, Koehorst, H.J.J., Bruinsma, T., Jansen, J., Custers, J., De Jong J. and Dons, J.J.M. (1992) Adventitious shoot formation fromin vitro leaf explants of carnation (Diathus caryophyllus L.).Sci. Hortic. 51, 223–35.
Van Altvorst, A.C. van, Koehorst, H.J.J., Bruinsma, T. and Dons, J.J.M. (1994) Improvement of adventitious shoot formation from carnation leaf explants.Pl. Cell, Tiss. Organ Culture 37, 87–90.
Van der Beek, J.G. van der, Verkerk, R., Zabel, P. and Lindhout, P. (1992) Mapping strategy for resistance genes in tomato based on RFLPs between cultivars:Cf9 (resistance toCladosporium fulvum) on chromosome 1.Theor. Appl. Genet. 84, 106–12.
Van Wordragen, M. van and Dons, J.M.M. (1992)Agrobacterium tumefaciens mediated transformation of recalcitrant crops; a review.Pl. Mol. Biol. Rep. 10, 12–36.
Yenofsky, R.L., Fine, M.F. and Pellow, J.W. (1990) A mutant neomycin phosphotransferase II gene reduces the resistance of transformants to antibiotic selection pressure.Proc. Natl Acad. Sci. USA 87, 3435–9.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
van Altvorst, AC., Riksen, T., Koehorst, H. et al. Transgenic carnations obtained byAgrobacterium tumefciens-mediated transformation of leaf explants. Transgenic Research 4, 105–113 (1995). https://doi.org/10.1007/BF01969412
Received:
Revised:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF01969412