Abstract
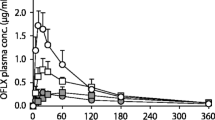
A study was conducted to evaluate the pharmacokinetics of loracarbef, a new synthetic oral carbacephem antibiotic, following administration of 400 mg in normal male volunteers. The influence of food and possible interaction with acetylcysteine, a commonly used mucolytic agent, was also studied. Twelve healthy volunteers participated in the study and randomly received an oral dose of 400 mg loracarbef in the fasting state, 400 mg loracarbef following a standard breakfast or 400 mg loracarbef together with 200 mg acetylcysteine in granular form. Serum and urine concentrations were determined over 24 h by means of a bioassay. Loracarbef was well tolerated. Four volunteers complained of mild, transient headache. The substance was well absorbed with a mean peak level of 19.21±3.94 mg/l in the fasting state; it was primarily excreted in active form via the kidneys (urine recovery/24 h: 86–92 %). The elimination half-life ranged from 70.3 to 102.0 min. Acetylcysteine had no effect on the absorption of loracarbef. The intake together with food delayed the absorption time, but had no influence on the bioavailability.
Similar content being viewed by others
References
Cao C, Chin NX, Neu HC In vitro activity and beta-lactamase stability of LY163892. Journal of Antimicrobial Chemotherapy 1988, 22: 155–165.
Howard AJ, Dunkin KT Comparative in vitro activity of a new carbacephem, LY163892. Journal of Antimicrobial Chemotherapy 1988, 22: 445–456.
Jones RN, Barry AL Antimicrobial activity of LY163892, an orally administered 1-carbacephem. Journal of Antimicrobial Chemotherapy 1988, 22: 315–320.
Knapp CC, Washington JA In vitro activities of LY163892, cefaclor, and cefuorxime. Antimicrobial Agents and Chemotherapy 1988, 32: 131–133.
Pelosi E, Fontana R In vitro activity and beta-lactamase stability of LY163892. European Journal of Clinical Microbiology and Infectious Diseases 1988, 8: 549–551.
Shelton S, Nelson JD In vitro susceptibilities of common pediatric pathogens to LY163892. Antimicrobial Agents and Chemotherapy 1988, 32: 268–270.
Reeves DS, Bywater MJ Assay of antimicrobial agents. In: de Louvois J (ed): Selected topics in clinical bacteriology. Bailliere Tyndall, London, 1976, p. 21–78.
Schwarz G Estimating the dimension of a model. Annals of Statistics 1986, 6: 461–464.
Benet L Effect of route of administration and distribution of drug action. Journal of Pharmacokinetics and Biopharmaceutics 1978, 6: 559–585.
Bennett JV, Brodie JL, Benner EJ, Kirby MM Simplified, accurate method for antibiotic assay of clinical specimens. Applied Microbiology 1966, 14: 170–177.
Greenblatt D, Koch-Weser J Clinical pharmacokinetics. New England Journal of Medicine 1975, 193: 702–705.
Koeppe P A new regression function for absorption kinetics. Arzneimittel Forschung 1988, 38: 1375–1377.
Ritschel WA Handbook of basic pharmacokinetics. Drug Intelligence Publications, Hamilton, 1986, p. 269–301.
Gibaldi M Biopharmaceutics and clinical pharmacokinetics. Lea & Febiger, Philadelphia, 1991, p. 409–416.
Nakashima M, Uematsu T, Takigutchi Y, Mitsuno A, Iida M, Yoshida T, Yamamoto S, Kitagawa K, Oguma T, Ishii H, Yamada H Phase I clinical studies of 7432-S, a new oral cephalosporin: safety and pharmacokinetics. Journal of Clinical Pharmacology 1988, 28: 246–252.
Tam YK, Kneer J, Dubach UC, Stoeckel K Pharmacokinetics of cefetamet pivoxil with ascending oral doses in normal healthy volunteers. Antimicrobial Agents and Chemotherapy 1989, 33: 957–959.
Tam YK, Kneer J, Dubach UC, Stoeckel K Effects of timing of food and fluid volume on cefetamet pivoxil absorption in healthy normal volunteers. Antimicrobial Agents and Chemotherapy 1990, 34: 1556–1559.
Kees F, Naber G Pharmakokinetik von Cefixim bei Probanden und ein Literaturvergleich mit neuen Ester Prodrug Cephalosporinen. Infection 1990, 18, Supplement 3: 150–154.
Tanimura H, Kobayashi N, Saito T, Huang WF, Yoshido K Chemotherapy of bilary tract infections: concentrations of cefixime in bile and gallbladder tissue and clinical evaluation on bilary tract infections. Chemotherapy 1985, 33, Supplement 6: 499–517.
Welling PG, Tse FL The influence of food on the absorption of antimicrobial agents. Journal of Antimicrobial Chemotherapy 1982, 9: 7–27.
Stoeckel K, Tam YK, Kneer J Pharmacokinetics of oral cefetamet pivoxil and intravenous cefetamet in humans: a review. Current Medical Research and Opinion 1989, 11: 432–442.
Bonavita E Combined cefuroxime plus acetylcysteine in the treatment of bacterial infections of the respiratory tract. Drugs Under Experimental and Clinical Research 1985, 11: 361–368.
Girbino G, Giacobbe G, Ferrara F Physiopathological rationale and clinical aims of the use of a combination of cefuroxime and N-acetylcysteine in pneumology. International Journal of Clinical Pharmacology and Therapeutic Toxicology 1985, 23: 166–172.
Gottschalk B, Wichmann G Die Wirkung von N-Acetylcystein auf verschiedene Antibiotika. Das Deutsche Gesundheitswesen 1970, 15: 700–703.
Lawson D, Saggers BA Some observations on the penetration of antibiotics through mucus in vitro. Journal of Clinical Pathology 1966, 19: 313–317.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Roller, S., Lode, H., Stelzer, I. et al. Pharmacokinetics of loracarbef and interaction with acetylcysteine. Eur. J. Clin. Microbiol. Infect. Dis. 11, 851–855 (1992). https://doi.org/10.1007/BF01960891
Issue Date:
DOI: https://doi.org/10.1007/BF01960891