Abstract
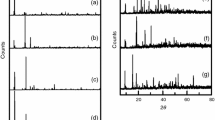
The multi-step dehydration and decomposition of trivalent lanthanide adipate hydrates has been investigated by TG, DTG and DTA, together with infrared study of these compounds and the corresponding intermediate decomposition products. X-ray diffraction data for adipate complexes of general stoichiometry Ln2(C6H8O4)3· 10H2O, where Ln=La, Ce, Pr, Nd, Sm, Eu, Gd, Tb, Dy, Ho, Er, Tm, Yb and Lu are also reported.
Zusammenfassung
Die in mehreren Schritten verlaufende Dehydratisierung und Zersetzung von Adipat-Hydraten der dreiwertigen Lanthanide wurden mittels TG, DTG und DTA untersucht. Diese Verbindungen und bei deren Zersetzung auftretende Zwischenprodukte wurden auch infrarotspektroskopisch charakterisiert. Röntgendiffraktometrische Daten sind für Adipatkomplexe der allgemeinen StöchiometrieLn2(C6H8O4)3·10H2O (Ln=La, Ce, Pr, Nd, Sm, Eu, Gd, Tb, Dy, Ho, Er, Tm, Yb und Lu) angegeben.
РЕжУМЕ
МЕтОДОМ тг, Дтг, ДтА И Ик спЕктРОскОпИИ ИжУЧЕ НА МпОгОстУпЕНЧАтАь ДЕ гИДРАтАцИь И РАжлОжЕНИЕ ДЕкАгИД РАтОВ лАНтАНОИДОВ АД ИпИНОВОИ кИслОты с ОБЩЕИ ФОРМУ лОИ Ln2(C6H8O4)3·10Н2О. МЕтОД Ик спЕ ктРОскОпИИ Был ИспОльжОВАН тАкжЕ Дл ь ИДЕНтИФИкАцИИ пРОМЕжУтОЧНых пРОДУ ктОВ РАжлОжЕНИь. Дль В сЕх ИсхОДНых сОлЕИ пРЕДс тАВлЕНы тАкжЕ ДАННыЕ РЕНтгЕНОстРУктУРНО гО АНАлИжА.
Similar content being viewed by others
References
B. Wunderlich and R. C. Bopp, J. Thermal Anal., 6 (1974) 335.
B. D. Jain, Current Science, India, 32 (1963) 66.
D. J. Riabchikov and E. A. Terentieva, Izv. Acad. Nauk USSR, Otd. Khim. Neorg., 1 (1949) 44.
J. M. Peacook and J. C. James, J. Chem. Soc., (1951) 2233.
J. M. Korenman and D. N. Sokolov, Trudy po Khimii i Khimich. Tekhnol., 2 (1961) 311; Ref. Zh. Khim., (USSR), 1961, 12B 543.
D. N. Shelke and D. W. Jahagirdar, Bull. Chem. Soc. Japan, 49 (1976) 2442.
W. Brzyska and W. Hubicki, Ann. Univ. M. Curie-Skłodowska, Lublin, Sect. AA 23 (1968) 77; Chem. Abstr., 71 (1969) 116998 b.
B. S. Azikov and W. W. Serebrennikov, Trudy Tomsk. Gos. Univ. Ser. Khim., 185 (1965) 111; Chem. Abstr., 66 (1967) 43328 t.
B. S. Azikov and W. W. Serebrennikov, ibid., 185 (1965) 118; Chem. Abstr., 66 (1967) 43327 s.
B. S. Azikov and W. W. Serebrennikov, ibid., 192 (1968) 71; Chem. Abstr., 73 (1970) 72665 a.
A. M. Wynne and J. E. Roberts, Thermochim. Acta, 7 (1973) 159.
A. J. Vogel, J. Chem. Soc., (1929) 722.
K. Nakamoto, Infrared Spectra of Inorganic and Coordination Compounds, J. Wiley, New York-London 1963.
J. Loners and N. Caro, Journ. des Recherches du CNRS, 9 (1957) 107.
W. W. Wendlandt, Analyt. Chem., 30 (1958) 58; 31 (1959) 408.
M. Dąbkowska, J. Polish Chem. (in press).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Dąbkowska, M. A thermal analysis study of lanthanide adipate hydrates. Journal of Thermal Analysis 32, 71–77 (1987). https://doi.org/10.1007/BF01914549
Received:
Revised:
Issue Date:
DOI: https://doi.org/10.1007/BF01914549